|
|

| Biomarker & Treatment for Heart Failure |
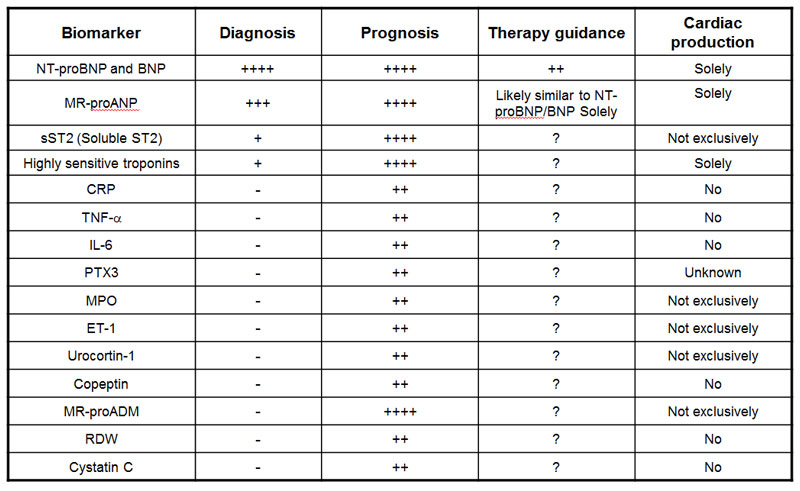
Roland R.J., van Kimmenade RR, James L., Clin Chem. 2012 Jan;58(1):127-38. doi: 10.1373/clinchem.2011.165720. Epub 2011 Nov 15.
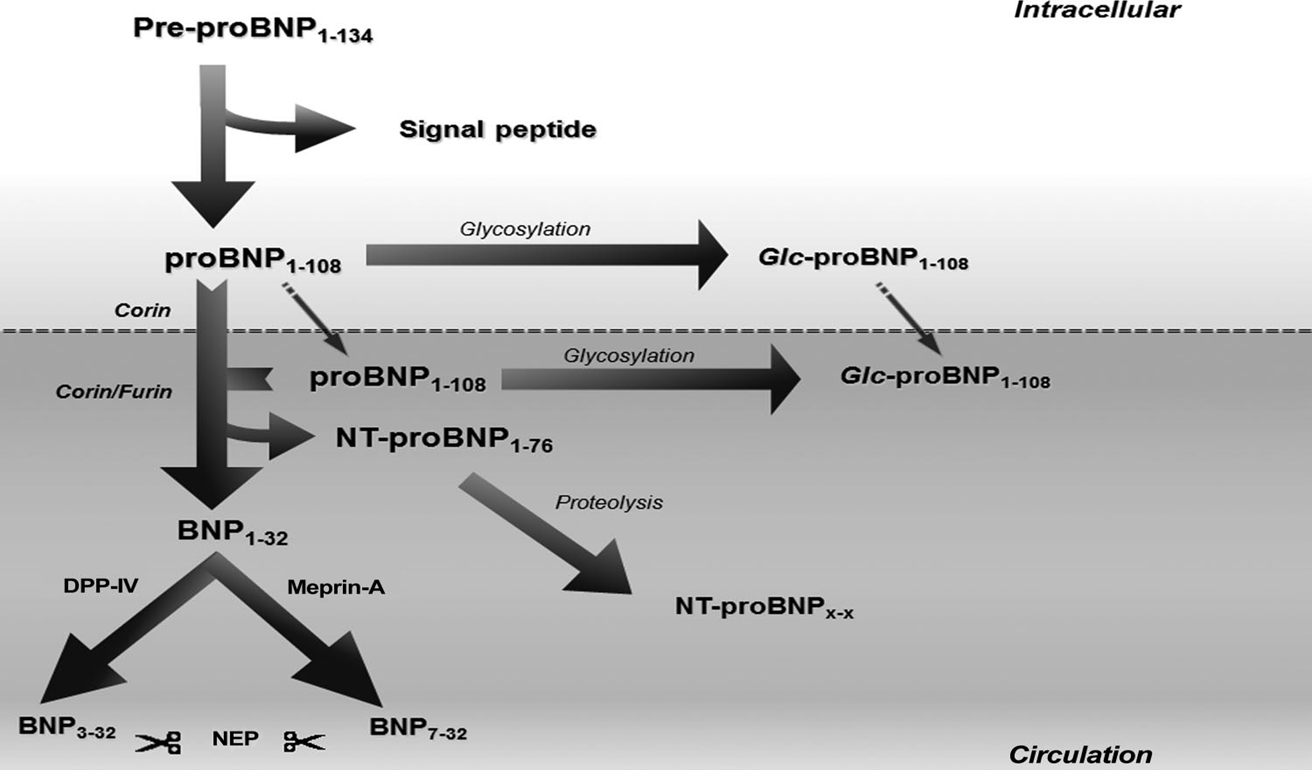
Abbreviation: Glc, glycosylated; NEP, neutral endopeptidase; DPP-IV, dipeptidyl peptidase-IV.
Ridker el al., N Engl J Med 2002:347: 1557
Tchemof et al., Circulation 2002; 105: 564
 |
BACKGROUND:
Serelaxin, recombinant human relaxin-2, is a vasoactive peptide hormone with many biological and haemodynamic effects. In a pilot study, serelaxin was safe and well tolerated with positive clinical outcome signals in patients with acute heart failure. The RELAX-AHF trial tested the hypothesis that serelaxin-treated patients would have greater dyspnoea relief compared with patients treated with standard care and placebo.
METHODS:
RELAX-AHF was an international, double-blind, placebo-controlled trial, enrolling patients admitted to hospital for acute heart failure who were randomly assigned (1:1) via a central randomisation scheme blocked by study centre to standard care plus 48-h intravenous infusions of placebo or serelaxin (30 μg/kg per day) within 16 h from presentation. All patients had dyspnoea, congestion on chest radiograph, increased brain natriuretic peptide (BNP) or N-terminal prohormone of BNP, mild-to-moderate renal insufficiency, and systolic blood pressure greater than 125 mm Hg. Patients, personnel administering study drug, and those undertaking study-related assessments were masked to treatment assignment. The primary endpoints evaluating dyspnoea improvement were change from baseline in the visual analogue scale area under the curve (VAS AUC) to day 5 and the proportion of patients with moderate or marked dyspnoea improvement measured by Likert scale during the first 24 h, both analysed by intention to treat. This trial is registered at ClinicalTrials.gov, NCT00520806.
FINDINGS:
1161 patients were randomly assigned to serelaxin (n=581) or placebo (n=580). Serelaxin improved the VAS AUC primary dyspnoea endpoint (448 mm × h, 95% CI 120-775; p=0.007) compared with placebo, but had no significant effect on the other primary endpoint (Likert scale; placebo, 150 patients [26%]; serelaxin, 156 [27%]; p=0.70). No significant effects were recorded for the secondary endpoints of cardiovascular death or readmission to hospital for heart failure or renal failure (placebo, 75 events [60-day Kaplan-Meier estimate, 13.0%]; serelaxin, 76 events [13.2%]; hazard ratio [HR] 1.02 [0.74-1.41], p=0.89] or days alive out of the hospital up to day 60 (placebo, 47.7 [SD 12.1] days; serelaxin, 48.3 [11.6]; p=0.37). Serelaxin treatment was associated with significant reductions of other prespecified additional endpoints, including fewer deaths at day 180 (placebo, 65 deaths; serelaxin, 42; HR 0.63, 95% CI 0.42-0.93; p=0.019).
INTERPRETATION:
Treatment of acute heart failure with serelaxin was associated with dyspnoea relief and improvement in other clinical outcomes, but had no effect on readmission to hospital. Serelaxin treatment was well tolerated and safe, supported by the reduced 180-day mortality.
Remark:
Serelaxin (recombinant human relaxin-2 ) might be a breakthrough in the treatment of acute, decompensated HF and, after the RELAX?AHF trial, Relaxin is the first drug that seems to improve both morbidity and mortality in patients with acute, decompensated HF.
Teerlink JR et al., Lancet. 2013 Jan 5;381(9860):29-39. doi: 10.1016/S0140-6736(12)61855-8. Epub 2012 Nov 7.
|
 |
OBJECTIVES:
The aim of this study was to assess the effects of serelaxin on short-term changes in markers of organ damage and congestion and relate them to 180-day mortality in patients with acute heart failure.
BACKGROUND:
Hospitalization for acute heart failure is associated with high post-discharge mortality, and this may be related to organ damage.
METHODS:
The Pre-RELAX-AHF (Relaxin in Acute Heart Failure) phase II study and RELAX-AHF phase III study were international, multicenter, double-blind, placebo-controlled trials in which patients hospitalized for acute heart failure were randomized within 16 h to intravenous placebo or serelaxin. Each patient was followed daily to day 5 or discharge and at days 5, 14, and 60 after enrollment. Vital status was assessed through 180 days. In RELAX-AHF, laboratory evaluations were performed daily to day 5 and at day 14. Plasma levels of biomarkers were measured at baseline and days 2, 5, and 14. All-cause mortality was assessed as a safety endpoint in both studies.
RESULTS:
Serelaxin reduced 180-day mortality, with similar effects in the phase II and phase III studies (combined studies: N = 1,395; hazard ratio: 0.62; 95% confidence interval: 0.43 to 0.88; p = 0.0076). In RELAX-AHF, changes in markers of cardiac (high-sensitivity cardiac troponin T), renal (creatinine and cystatin-C), and hepatic (aspartate transaminase and alanine transaminase) damage and of decongestion (N-terminal pro-brain natriuretic peptide) at day 2 and worsening heart failure during admission were associated with 180-day mortality. Serelaxin administration improved these markers, consistent with the prevention of organ damage and faster decongestion.
CONCLUSIONS:
Early administration of serelaxin was associated with a reduction of 180-day mortality, and this occurred with fewer signs of organ damage and more rapid relief of congestion during the first days after admission.
Metra M et al, J Am CollCardiol. 2013 Jan 15;61(2):196-206. doi: 10.1016/j.jacc.2012.11.005.
|

|
OBJECTIVE:
Primarily to develop a multimarker score for prediction of 3-year mortality in older patients with decompensated heart failure (HF).
DESIGN:
Prospective cohort study.
SETTING:
Secondary care. Single centre. PATIENTS AND BIOMARKERS: 131 patients, aged ≥65 years, with decompensated HF were included. Assessment of biomarkers was performed at discharge.
PRIMARY OUTCOME MEASURE:
3-year mortality.
RESULTS:
Mean age was 73±11 years; mean left ventricular ejection fraction , 43±14%; 53% were male. The 3-year mortality was 53.4%. The following N-terminal brain natriuretic peptide (NTproBNP) levels could optimally stratify mortality: <2000 ng/l (n=39), 30.8% mortality; 2000-8000 ng/l (n=58), 51.7% mortality; and >8000 ng/l (n=34), 82.4% mortality. However, in the 2000-8000 ng/l range, NTproBNP levels had low-prognostic capacity, based on the area under the receiver operating characteristic curve (AUC=0.53; 95% CI 0.40 to 0.67). In this group, multivariate analysis identified age, cystatin C (CysC), and troponin T (TnT) levels as independent risk factors. A risk score based on these three risk factors separated a high-risk and low-risk groups within the NTproBNP range of 2000-8000 ng/l. The score exhibited a significantly higher AUC (0.75; 95% CI 0.62 to 0.86) than NTproBNP alone (p=0.03) in this NTproBNP group and had similar prognostic capacity as NTproBNP in patients below or above this NTproBNP range (p=0.57). Net reclassification improvement and integrated discriminatory improvement in the group with NTproBNP levels between 2000 and 8000 ng/l was 54% and 23%, respectively, and in the whole cohort 22% and 11%, respectively.
CONCLUSIONS:
Our results suggested that, to assess risk in HF, older patients required significantly higher levels of NTproBNP than younger patients. Furthermore, a risk score that included TnT and CysC at discharge, and age could improve risk stratification for mortality in older patients with HF in particular when NTproBNP was moderately elevated.
Bjurman C et al, BMJ Open. 2013 Mar 9;3(3). pii: e002254. doi: 10.1136/bmjopen-2012-002254. Print 2013.
|

|
Heart failure is the most frequent cause of hospitalization in elderly population. Unlike the therapy of congestive heart failure, there was only a modest progress in the medical treatment for acutely decompensated heart failure over the past several decades. Moreover, current treatment is associated with many limitations in clinical practice. The family of natriuretic peptides consists of several structurally similar polypeptides (ANP, BNP, CNP, urodilatin, DNP). ANP and BNP are the most characterized substances and represent an important compensatory mechanisms in heart failure because of their vasodilatory, natriuretic and antiproliferative effects. Nesiritide is a recombinant human BNP which has been shown to be effective in treating heart failure in several clinical trials. However, a recent meta-analysis revealed a nesiritide-associated increased 30-day-mortality rate. The results of initial small-sized trials suggest beneficial hemodynamic effects of urodilatin in decompensated heart failure. Despite of being approved for the treatment of decompensated heart failure in some countries, the clinical relevance of nesiritide is currently unclear. Urodilatin might represent a potential alternative.
Gassanov N et al, Dtsch Med Wochenschr. 2011 Aug;136(34-35):1738-43. doi: 10.1055/s-0031-1286068. Epub 2011 Aug 29.
|

|
AIMS:
Human stresscopin is a corticotropin-releasing factor (CRF) type 2 receptor (CRFR2) selective agonist and a member of the CRF peptide family. Stimulation of CRFR2 improves cardiac output and left ventricular ejection fraction (LVEF) in patients with stable heart failure (HF) with reduced LVEF. We examined the safety, pharmacokinetics, and effects on haemodynamics and serum biomarkers of intravenous human stresscopin acetate (JNJ-39588146) in patients with stable HF with LVEF ≤35% and cardiac index (CI) ≤2.5 L/min/m2.
METHODS AND RESULTS:
Sixty-two patients with HF and LVEF ≤35% were instrumented with a pulmonary artery catheter and randomly assigned (ratio 3:1) to receive an intravenous infusion of JNJ-39588146 or placebo. The main study was an ascending dose study of three doses (5, 15, and 30 ng/kg/min) of study drug or placebo administered in sequential 1 h intervals (3 h total). Statistically significant increases in CI and reduction in systemic vascular resistance (SVR) were observed with both the 15 ng/kg/min (2 h time point) and 30 ng/kg/min (3 h time point) doses of JNJ-39588146 without significant changes in heart rate (HR) or systolic blood pressure (SBP). No statistically significant reductions in pulmonary capillary wedge pressure (PCWP) were seen with any dose tested in the primary analysis, although a trend towards reduction was seen.
CONCLUSION:
In HF patients with reduced LVEF and CI, ascending doses of JNJ-39588146 were associated with progressive increases in CI and reductions in SVR without significant effects on PCWP, HR, or SBP.Trial registration: NCT01120210.
Gheorghiade M et al, Eur J Heart Fail. 2013 Mar 6. [Epub ahead of print]
|

|
BACKGROUND:
Until recently, biomarker testing in heart failure (HF) syndromes has been viewed as an elective supplement to diagnostic evaluation of patients suspected to suffer from this condition. This approach to the use of biomarker testing contrasts with other cardiovascular diagnoses such as acute myocardial infarction, for which biomarkers are integral to disease process definition, risk stratification, and in some cases treatment decision making.
CONTENT:
In this review we consider various perspectives on the evaluation of biomarkers in HF. In addition, we examine recent advances in the understanding of established biomarkers in HF (such as the natriuretic peptides), the elucidation of novel biomarkers potentially useful for the evaluation and management of patients with HF, and the growing understanding of important and relevant comorbidities in HF. We also review candidate biomarkers from a number of classes: (a) myocyte stretch, (b) myocyte necrosis, (c) systemic inflammation, (d) oxidative stress, (e) extracellular matrix turnover, (f) neurohormones, and (g) biomarkers of extracardiac processes, such as renal function.
SUMMARY:
Novel applications of established biomarkers of HF as well as elucidation and validation of emerging assays for HF syndromes have collectively led to a growing interest in the more widespread use of such testing in patients affected by the diagnosis.

van Kimmenade RR et al, Clin Chem. 2012 Jan;58(1):127-38. doi: 10.1373/clinchem.2011.165720. Epub 2011 Nov 15.
|

|
BACKGROUND:
We set out to evaluate the utility of selected heart failure (HF) biomarkers in patients with dilated cardiomyopathy (DCM).
METHODS:
In a prospective, randomized study, 68 DCM patients with left ventricular ejection fraction (LVEF) ≤40% treated optimally were included. They were observed for 5years. Initial and control tests included full clinical examination, measurement of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and IL-10, syndecan-4, cystatin C (CysC) and N-terminal pro-brain natriuretic peptide (NT-proBNP), echocardiographic examination, and the assessment of exercise capacity in 6-minute walk test (6MWT).
RESULTS:
Finally, after 5-year follow-up we analyzed the data of 45 DCM patients. Concentration of syndecan-4 correlated negatively with LVEF (R=-0.36, p=0.02) and positively with LV systolic (R=0.57, p<0.001) and diastolic diameters (R=0.64, p<0.001). A positive correlation between CysC and right ventricular diastolic diameter (R=0.38, p=0.01), and negative correlations between CysC and glomerular filtration rate (R=-0.49, p<0.001), LVEF (R=-0.4, p=0.02), and 6MWT (R=-0.46, p<0.001) were noted. Patients who had an increase in LVEF during 5years were characterized by lower levels of CysC (p=0.01) and NT-proBNP (p<0.001). CysC≤95mg/l and NT-proBNP≤32pg/ml were the best predictors of LVEF increase in DCM patients. Multivariate regression analysis showed that 6MWT was the only independent predictor of HF re-hospitalization (OR 0.989; p<0.001), and NT-proBNP and LV diastolic diameter were the only risk factors of increased mortality (OR 1.001; p=0.007 and OR 2.960; p=0.025, respectively) in DCM patients.
CONCLUSIONS:
CysC correlates negatively with both kidney function and exercise capacity. Syndecan-4 may be a useful biomarker for predicting adverse LV remodeling in DCM patients.
Bielecka-Dabrowa A et al, Int J Cardiol. 2013 Feb 14. pii: S0167-5273(13)00199-X. doi: 10.1016/j.ijcard.2013.01.157. [Epub ahead of print] |

|
Heart failure (HF) biomarkers have dramatically impacted the way HF patients are evaluated and managed. B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) are the gold standard biomarkers in determining the diagnosis and prognosis of HF, and studies on natriuretic peptide-guided HF management look promising. An array of additional biomarkers has emerged, each reflecting different pathophysiological processes in the development and progression of HF: myocardial insult, inflammation and remodeling. Novel biomarkers, such as mid-regional pro atrial natriuretic peptide (MR-proANP), mid-regional pro adrenomedullin (MR-proADM), highly sensitive troponins, soluble ST2 (sST2), growth differentiation factor (GDF)-15 and Galectin-3, show potential in determining prognosis beyond the established natriuretic peptides, but their role in the clinical care of the patient is still partially defined and more studies are needed. This article is part of a Special Issue entitled: Heart failure pathogenesis and emerging diagnostic and therapeutic interventions.
Gaggin HK, Januzzi JL Jr, BiochimBiophysActa. 2013 Jan 9. pii: S0925-4439(13)00009-4. doi: 10.1016/j.bbadis.2012.12.014. [Epub ahead of print]
|
|
|
|
relaxin
%Relaxin 2%;%periostin%;%PEDF%;%Stresscopin%
|
|
|


