|
|
| Obestatin |
| A New Physiological Opponent of Ghrelin |
|
The ghrelin-motilin receptor family and their ligands. Each of these gastrointestinal hormones acts on a specific G protein-coupled receptor from the same family to affect food intake and gastrointestinal
motility (9-11). Similar dual effects on satiety and gastrointestinal motility are known for glucagon-like peptide 1, cholecystokinine, or peptide YY. Collectively, these peptides may serve to couple meal termination
with inhibition of upper gastrointestinal function to prevent ma labsorption and postprandial metabolic disturbances (1, 2, 8).
Ruben Nogueiras and Matthias Tschöp. Science, Vol 310, Issue 5750, 985-986 , 11 November 2005
|

BACKGROUND:: The aim was to determine obestatin and ghrelin serum levels and their ratio in inflammatory bowel disease (IBD) patients. METHODS:: We measured the ghrelin and obestatin levels of 31 Crohn's disease patients and 22 patients with ulcerative colitis using a radioimmunoassay method. Circulating levels of the 2 hormones and their ratio were correlated with the disease type and activity, disease localization, and treatment. RESULTS:: The mean ghrelin value was statistically significantly higher in patients with active disease (402.4 +/- 462.6 pg/mL) than in patients in remission (148.2 +/- 59.6 pg/mL) P = 0.0290, alpha = 0.05, whereas obestatin mean values were not (217.4 +/- 59.8 pg/mL in active disease and 189.0 +/- 46.8 pg/mL in patients with inactive disease P = 0.0607). When we evaluated the obestatin/ghrelin ratio between active and inactive disease, it was found that the ratio in active disease was statistically significantly lower (0.8 +/- 0.3) than in patients in remission (1.4 +/- 0.3) P < 0.001, alpha = 0.05. There is also a statistically significantly correlation between obestatin/ghrelin ratio and disease activity (P < 0,001). CONCLUSIONS:: Ghrelin and obestatin seem to play a significant role in IBD pathogenesis. Further studies are needed to elucidate the role of these hormones as new biological markers of activity of IBD. Inflamm Bowel Dis 2009.
Alexandridis et al. Inflamm Bowel Dis. 2009 Apr 30. [Epub ahead of print]
Background Obestatin
is a novel hormone that is encoded by the Ghrelin gene and produced in
the gut. Ghrelin is profoundly orexogenic and adipogenic, increasing
food intake and body weight. This new ghrelin-associated peptide behaves
as a physiological opponent of ghrelin in rodent animals, but its pathophysiological
role in humans remains unknown Objective In this study we investigate
whether plasma obestatin level is different in patients with impaired
glucose regulation (IGR) and type 2 diabetes mellitus (T2DM). Patients
and measurements Forty-seven patients with T2DMu, 30 subjects with IGR,
and 38 sex- and age-matched normal controls participated in the study.
Plasma obestatin levels were measured with a radioimmunoassay (Phoenix
Pharmaceuticals, Inc.). The relationship between plasma obestatin levels
and anthropometric and metabolic parameters was also analysed. Results
Plasma obestatin levels were lower in patients with T2DM and IGR than
in controls (37.5 +/- 9.2 ng/l and 39.2 +/- 9.7 ng/l vs. 43.8 +/- 8.0
ng/l, P = 0.002 and P = 0.039, respectively). Decreasing concentrations
of obestatin were independently and significantly associated with IGR
and T2DM. Multiple logistic regression analysis revealed obestatin to
be independently associated with IGR and T2DM. In a multiple linear regression
analysis, only waist-to-hip ratio and homeostasis model assessment of
insulin resistance (HOMA-IR) were independently associated with plasma
obestatin level. Conclusion Our results suggest that obestatin may play
a role in appetite regulation in patients with IGR and T2DM.
Qi X, Li L, Yang G, Liu J, Li K, Tang Y, Liou H, Boden G. Clin Endocrinol (Oxf). 2007 Apr;66(4):593-7
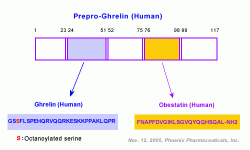
Context: Obestatin,
a sibling of ghrelin derived from preproghrelin, opposes the ghrelin's
effects on food intake. Plasma obestatin profiles in relation to ghrelin
have not been fully investigated in human obesity. Objective: We hypothesize
that obesity might present with imbalance of circulating ghrelin and
obestatin levels. Setting: In-patient department of Changhai Hospital,
Shanghai, China. Participants: Sixteen obese (8 men, aged 58.8+/-4.9;
8 women, aged 59.9+/-9.6) and fourteen normal weight individuals (7 men,
aged 52.7+/-5.9; 7 women, aged 56.1+/-4.9). Main Outcome Measures: Total
plasma ghrelin and obestatin levels, one hour before and two hours after
breakfast, were measured by radioimmunoassay [from Phoenix Biotech (Beijing)].
Results: Both preprandial plasma ghrelin levels (P < 0.01) and obestatin
levels (P < 0.01) were lower in the obese compared with normal weight
controls. However, unexpectedly, the ratio of preprandial ghrelin to
obestatin was higher in obese compared with normal weight controls (P <
0.01) even after adjustment for gender and age (P < 0.01). The ratio
of postprandial ghrelin to obestatin was decreased both in obese (P < 0.05)
and controls (P < 0.01) compared with their preprandial levels. There
were no significant differences in the ratio of postprandial ghrelin to
obestatin between obese and normal weight controls. BMI was positively
correlated with and a significantly independent determinant of the preprandial
ghrelin to obestatin ratio. Conclusion: Circulating preprandial ghrelin
to obestatin ratio is elevated in human obesity. We suggest that high preprandial
ghrelin to obestatin ratio may be involved in the etiology and pathophysiology
of obesity.
Guo ZF, Zheng X, Qin YW, Hu JQ, Chen SP, Zhang Z. J Clin Endocrinol Metab. 2007 Feb 13; [Epub ahead of print]
Prader-Willi syndrome (PWS) is an obesity syndrome characterized by rapid weight
gain and excessive food intake. Food intake is regulated by the hypothalamus
but directly influenced by gastrointestinal peptides responding to
the nutritional status and body composition of an individual. Ghrelin,
derived from preproghrelin, is secreted by the stomach and increases
appetite while obestatin, a recently identified peptide derived post-translationally
from preproghrelin, works in opposition to ghrelin by decreasing appetite.
The objective of this study was to measure fasting obestatin and ghrelin
levels in peripheral blood of subjects with PWS and compare to age
and gender matched control subjects. Plasma obestatin and ghrelin levels
were measured in subjects with PWS (n = 16, mean age = 16.0 +/- 13.3
years; age range 1-44 years) and age and gender matched control subjects
(n = 16). Significantly higher obestatin levels were seen in the 16
PWS subjects (398 +/- 102 pg/ml) compared with 16 controls (325 +/-
109 pg/ml; matched t-test, P = 0.04), particularly in 5 young (</=3
years old) PWS subjects (460 +/- 49 pg/ml) compared with 5 young controls
(369 +/- 96 pg/ml; matched t-test, P = 0.03). No significant difference
in ghrelin levels was seen between the PWS and comparison groups. No
significant correlation was observed for either peptide when compared
with body mass index but a significant negative correlation was seen
for ghrelin and age in PWS subjects. Our observations suggest that
obestatin may be higher in infants with PWS compared to comparison
infants. The possibility that obestatin may contribute to the failure
to thrive which is common in infants with PWS warrants further investigation.
Butler MG, Bittel DC. Am J Med Genet A. 2007 Mar 1;143(5):415-21.
Obestatin is a recently discovered peptide hormone that appears to be involved in reducing food
intake, gut motility and body weight. Obestatin is a product of the preproghrelin
gene and appears to oppose several physiological actions of ghrelin.
This study investigated the acute effects of obestatin (1-23) and the
truncated form, obestatin (11-23), on feeding activity, glucose homeostasis
or insulin secretion. Mice received either intraperitoneal obestatin
(1-23) or (11-23) (1mumol/kg) 4h prior to an allowed 15min period of
feeding. Glucose excursions and insulin responses were lowered by 64-77%
and 39-41%, respectively, compared with saline controls. However this
was accompanied by 43% and 53% reductions in food intake, respectively.
The effects of obestatin peptides were examined under either basal or
glucose (18mmol/kg) challenge conditions to establish whether effects
were independent of changes in feeding. No alterations in plasma glucose
or insulin responses were observed. In addition, obestatin peptides had
no effect on insulin sensitivity as revealed by hypoglycaemic response
when co-administered with insulin. Our observations support a role for
obestatin in regulating metabolism through changes of appetite, but indicate
no direct actions on glucose homeostasis or insulin secretion.
Peptides. 2007 Feb 12; [Epub ahead of print]
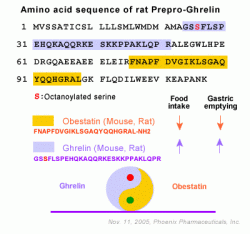
Obestatin, a 23 amino acid peptide recently isolated from the rat stomach, is encoded by the same gene that encodes ghrelin. With
the use of an antiserum directed against the mouse/rat obestatin, obestatin
immunoreactivity (irOBS) was detected in cells of the gastric mucosa,
myenteric plexus, and in Leydig cells of the testis in Sprague-awley
rats. Double labeling the myenteric plexus with obestatin antiserum and choline acetyltransferase (ChAT)
antiserum revealed that nearly all irOBS neurons were ChAT positive
and vice versa. For comparative purposes, myenteric ganglion cells,
cells in the gastric mucosa, and Leydig cells of the testis were shown
to be immunoreactive to preproghrelin. The biological activity of obestatin
on rat central neurons was assessed by the calcium microfluorimetric
Fura-2 method. Obestatin (100 nM) administered to dissociated and cultured
rat cerebral cortical neurons elevated cytosolic calcium concentrations
[Ca2C]i in a population of cortical neurons. The result provides the
first immunohistochemical evidence that obestatin is expressed in cells
of the gastric mucosa and myenteric ganglion cells, and also in Leydig
cells of the testis; the peptide is biologically active on central
neurons.
Siok L Dun, G Cristina Brailoiu, Eugen Brailoiu, Jun Yang1, Jaw Kang Chang1 and Nae J Dun
Q1 Department of Pharmacology, Temple University School of Medicine, 3420
N. Broad Street, Philadelphia, Pennsylvania 19140, USA
1Phoenix Pharmaceuticals Inc., Belmont, California 94002, USA;
Journal of Endocrinology (2006) 191, 1?0
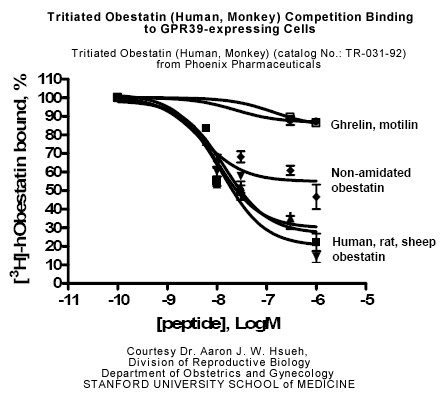
Derived from the same prohormone, obestatin has been reported to exert effects on food
intake that oppose those of ghrelin. The obestatin receptor, GPR39, is
present in brain and pituitary gland. Since the gene encoding those two
peptides is expressed also in those tissues, we examined further the
possible actions of obestatin in vivo and in vitro. Intracerebroventricular
administration of obestatin inhibited water drinking in ad libitum fed
and watered rats, and in food and water deprived animals. The effects
on water drinking preceded and were more pronounced than any effect on
food intake, and did not appear to be the result of altered locomotor/behavioral
activity. In addition, obestatin inhibited angiotensin II-induced water
drinking in animals provided free access to water and food. Current clamp
recordings from cultured, subfornical organ neurons revealed significant
effects of the peptide on membrane potential suggesting this as a potential
site of action. In pituitary cell cultures, log molar concentrations
of obestatin ranging from 1.0 pM to100 nM failed to alter basal growth
hormone (GH) secretion. In addition, 100 nM obestatin failed to interfere
with the stimulation of GH secretion by GH-releasing hormone or ghrelin,
and did not alter the inhibition by somatostatin in vitro. We conclude
that obestatin does not act in pituitary gland to regulate GH secretion,
but may act in brain to alter thirst mechanisms. Importantly, in rats
the effects of obestatin on food intake may be secondary to an action
of the peptide to inhibit water drinking. Key words: Ghrelin, Obestatin,
Thirst, Appetite, Growth Hormone.
Samson WK, White MM, Price C, Ferguson AV. Am J Physiol Regul Integr Comp Physiol. 2006 Aug 24; [Epub ahead of print]
 |
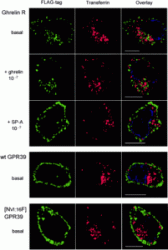
Internalization properties of the ghrelin receptor and of GPR39. Internalization was studied by antibody feeding experiments performed for 45 min with the M2 antibody in HEK293 cells stably transfected
with either the FLAG-tagged version of the ghrelin receptor (top three rows), GPR39, or [AsnVI:16Phe]GPR39 (bottom two rows). After fixation and permeabilization, the localization of the M2 antibody was done
with an Alexa 488-labeled second antibody (green). Cells were co-exposed to Texas Red-labeled transferrin, which is constitutively internalized by the ubiquitous transferrin receptor. The immunofluorescent
analysis was performed on a Zeiss Axiovert 100 microscope, in which out of focus light was deconvoluted from stacks of vertical images and a central 3.0-µm z-section was reconstructed using Improvision's
Volocity software (see"Experimental Procedures"). These sections, viewed from above, are illustrated for representative single cells from three independent experiments. The ghrelin receptor was exposed to either the agonist,
10-7 M ghrelin (second row), or the inverse agonist, 10-6 M [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-substance P (third row) for the last 15 min of the incubation period.
Holst B, et al. J Biol Chem. 2004 Dec 17;279(51):53806-17. Epub 2004 Sep 21.
|
|
|
Mutational analysis of the ghrelin receptor, with focus on the inner faces of the extracellular ends of TMs III, VI, and VII. A, helical wheel diagram of the ghrelin receptor. Residues that could be mutated
without eliminating the constitutive activity are indicated in black on gray. Two addition residues (Glu-196 and Arg-198) in extracellular loop 2 located on either side of the Cys residue that forms a disulfide
bridge to CysIII:01 were also substituted because they are believed to be located just above the main ligand binding pocket. In white on black are the three Phe residues that were subjected to more elaborate mutagenesis plus the two other residues, GlnIII:05 and ArgVI:20,
which were also hits for constitutive activity. B, inositol phosphate accumulation in COS-7 cells transiently transfected with the wild-type (WT) and a series of mutant forms of the ghrelin receptor. Black columns
indicate the basal, constitutive signaling activity, and the open bars represent the signaling activity in response to the agonist ghrelin (10-7 M) expressed as percentage of wild-type ghrelin receptor.
Holst B, et al. J Biol Chem. 2004 Dec 17;279(51):53806-17. Epub 2004 Sep 21.
|
|

|
Molecular model of the residues proposed to be part of the structural basis for the high constitutive activity in the ghrelin receptor. A, molecular model of the ghrelin receptor built over the inactive structure
of rhodopsin (60, 61). The seven helical bundle is displayed without the loops as viewed from the extra-cellular side. Only the residues on the inner faces of TMs III, VI, and VII, which in the mutational
analysis were identified to potentially be involved in the constitutive activity, are shown. The two arrows indicate the proposed inward movement of TM VI and VII toward TM III that occur during activation of 7TM
receptors based on metal-ion site engineering between residues: III:08, VI:16, and VII:06. B, the extracellular ends of TMs VI and VII as viewed from between TMs IV and V, showing the close proximity of the three
aromatic residues: PheVI:16, PheVII:06, and PheVII:09. These three residues were subject to further mutational analysis, as shown in Fig. 8.
Holst B, et al. J Biol Chem. 2004 Dec 17;279(51):53806-17. Epub 2004 Sep 21. |

Obestatin- and preproghrelin-immunoreactive cells in the rat myenteric plexus. (A) Several myenteric ganglia contain clusters of obestatin-immunoreactive cells. (B) Immunoreactivity is not detected in myenteric plexus processed with obestatin antiserum pre-absorbed with the peptide (1 mg/ml) overnight. (C) Several myenteric ganglia contain preproghrelin-immunoreactive cells of varying intensities. (D) A higher magnification of the area shown in C, where several preproghrelin-immunoreactive ganglion cells are clearly seen. Scale bar: A-C, 50 um and D, 25 um.
Dun, S. L. et al. Journal of Endocrinology (2006) 191, 1?0
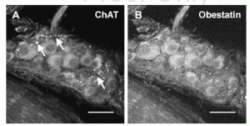
Confocal images of a rat myenteric ganglion double labeled with choline acetyltransferase- and obestatin antisera. (A) A group of myenteric ganglion cells exhibiting varying intensities of ChAT immunoreactivity. (B) The same group of
myenteric neurons labeled with obestatin antiserum. All the ganglion cells shown here contain both ChAT- and obestatin immunoreactivity; several strands of ChAT-immunoreactive fibers (arrows) seem to lack obestatin immunoreactivity.
Scale bar: 30 mm.
Dun, S. L. et al. Journal of Endocrinology (2006) 191, 1?0
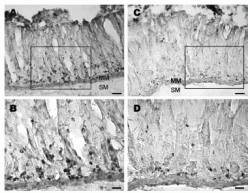
Longitudinal strips of rat stomach labeled with obestatin- or preproghrelin antiserum. (A) Obestatin-immunoreactive cells occur mostly in the glands of gastric mucosa. (B) A higher magnification
of the area in A where strongly labeled cells are seen in the glandular base. (C) Preproghrelin-immunoreactive cells are localized near the base of the glands. (D) A higher magnification
showing immunoreactive cells localized mainly to the glandular base. MM, muscularis mucosae and SM, submucosa. Scale bar: A and C, 100 mm; B and D, 25 mm.
Dun, S. L. et al. Journal of Endocrinology (2006) 191, 1?0
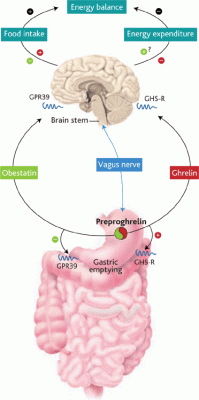
The Yin and Yang personalities of ghrelin and obestatin. Both hormones derive from the same precursor protein and are predominantly secreted by the stomach and released into the blood. Each acts on a
different receptor (GPR39 and GHS-R, as shown) and has an opposite
effect on food intake, body weight, and gastrointestinal motility.
Ruben Nogueiras and Matthias Tschöp. Science, Vol 310, Issue 5750, 985-986 , 11 November 2005
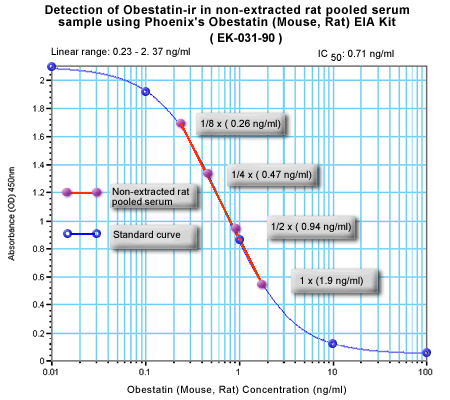 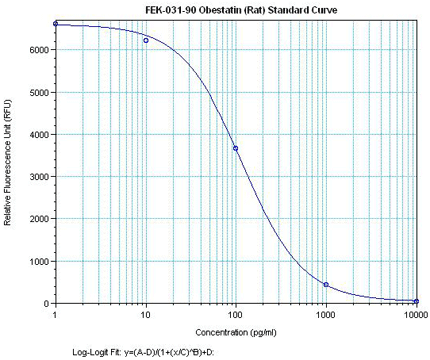
Minimum Detectable Concentration = 36.6 pg/ml
5.46 times more sensetive than normal EIA Kit

The ghrelin receptor family. A, schematic phylogenic tree of the ghrelin receptor family indicating the relative relationship of the
receptor. The black dots indicate the three receptors that either previously
(the ghrelin receptor and NT-R2 (8, 17)) or in the present study (GPR39)
have been demonstrated to display a high degree of constitutive signaling
activity. B, serpentine model of GPR39. Residues that are identical
among GPR39, the ghrelin receptor, and NT-R2 are highlighted in white
on black. The generic numbering system for 7TM receptor residues described
by Schwartz (59) is used throughout the article, and the proposed first
residues of each transmembrane helix are indicated by 1.
Holst B, et al. J Biol Chem. 2004 Dec 17;279(51):53806-17. Epub 2004 Sep 21.
Nogueiras R, et al. Endocrinology. 2006 Sep 28; [Epub ahead of print]
Zhang J. V., et al. Science, Vol 310, Issue 5750, 996-999 , 11 November 2005
Ruben Nogueiras and Matthias Tschöp. Science, Vol 310, Issue 5750, 985-986, 11 November 2005
|
|
|
ghrelin;ghrelin_c_terminal;des_oct_Ghrelin;061-19;MutateGhrelin;Ghrelin in tumor;031-22;ghrelin-receptor;ghrelin-cyto;ghrelin-determination;ghrelin-dap;Ghrelin-Reference-01;
%031-85%;%031-99%;%031-91%;%031-94%;%031-93%;%031-84%;%031-92%;%031-77%;%031-96%;%031-90%;%031-98%
|
|
|


