|
|
|
Bioactive Secretory Peptide/Protein Library (L-009)
|
|
Last updated:
|
A library covers various newly identified families secretory pro-hormones derived peptides that includes Chromogranin A (Vasostatin, Pancreastatin, Catestatin, Serpinin),Chromogranin B,VGF, Pro-SAAS, Secretogranin I, Secretogranin II , Secretogranin III, insulin-like growth factors (INSL 3, INSL 4, INSL 5, INSL 6, INSL 7 or relaxins), Prepro-ANP, Prepro-BNP, Prepro-CNP, proopiomelanocortin (POMC) derived peptides and Fractalkine (click underline to link each family)
More than 20 families of identified or suspected bioactive peptides enable a "reverse pharmacology approach" for large scale screening of bioactive peptides/proteins with more than over 1000 peptides/proteins which are HPLC-purified and fluorescein free.
Library Format :
 |
Packaged in 96 well plates |
 |
1.5 nanomoles of peptide in 15 micrograms BSA |
|
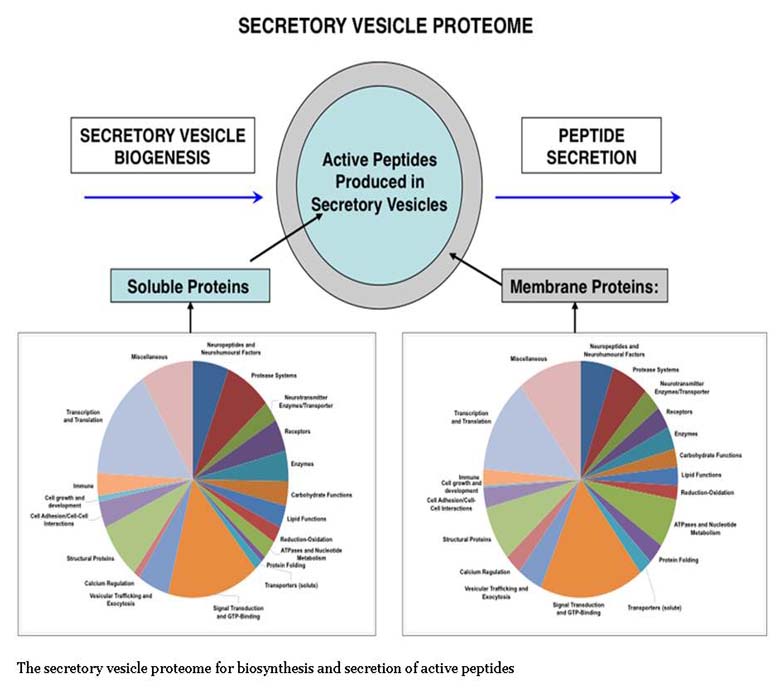
Figure From: Hook V et al., Biochim Biophys Acta. 2012 Jan;1824(1):89-104. doi: 10.1016
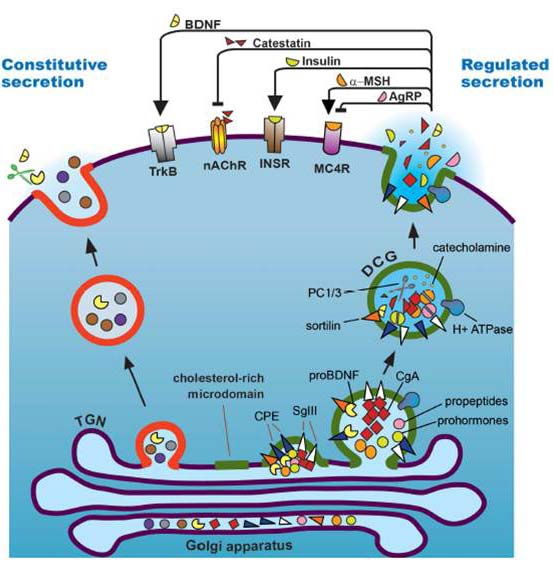
| Regulated secretory pathway of a model neuroendocrine cell. Propeptides and prohormones are packaged into dense core granules (DCGs), where they frequently undergo tissue-specific processing as the DCG matures. Proteins of the granin family are DCG components, and although their function is not fully understood, data suggest they are involved in DCG formation and regulated protein/peptide secretion, in addition to their role as precursors of bioactive peptides.
Figure From: Wei-Jye Lin and Stephen R. Salton Front. Endocrinol., 06 August 2013 |
The chromogranins (chromogranin A and chromogranin B), secretogranins (secretogranin II and secretogranin III), and additional related proteins (7B2, NESP55, proSAAS, and VGF) that together comprise the granin family subserve essential roles in the regulated secretory pathway that is responsible for controlled delivery of peptides, hormones, neurotransmitters, and growth factors. Here we review the structure and function of granins and granin-derived peptides and expansive new genetic evidence, including recent single-nucleotide polymorphism mapping, genomic sequence comparisons, and analysis of transgenic and knockout mice, which together support an important and evolutionarily conserved role for these proteins in large dense-core vesicle biogenesis and regulated secretion. Recent data further indicate that their processed peptides function prominently in metabolic and glucose homeostasis, emotional behavior, pain pathways, and blood pressure modulation, suggesting future utility of granins and granin-derived peptides as novel disease biomarkers.
Bartolomucci A1, Possenti R, Mahata SK, et al., Endocr Rev. 2011 Dec;32(6):755-97. doi: 10.1210/er.2010-0027. Epub 2011 Aug 23.
Chromogranin A (CgA), chromogranin B (CgB), and secretogranin II (SgII) belong to a family of uniquely acidic secretory proteins in elements of the diffuse neuroendocrine system. These "granins" are characterized by numerous pairs of basic amino acids as potential sites for intra- and extragranular processing. In response to adequate stimuli, the granins are coreleased with neurotransmitters and hormones and appear in the circulation as potential modulators of homeostatic processes. This review is directed towards functional aspects of the secreted CgA, CgB, and SgII and their biologically active sequences. Widely different effects and targets have been reported for granin-derived peptides. So far, the CgA peptides vasostatin-I, pancreastatin, and catestatin, the CgB peptides CgB(1-41) and secretolytin, and the SgII peptide secretoneurin are the most likely candidates for granin-derived regulatory peptides. Most of their effects fit into patterns of direct or indirect modulations of major functions, in particular associated with inflammatory conditions.
Helle KB. Results Probl Cell Differ. 2010;50:21-44. doi: 10.1007/400_2009_26.
Chromogranins (Cgs) constitute the main protein component in the vesicular matrix of large dense core vesicles (LDCV). These acidic proteins have been implicated in several physiological processes such as vesicle sorting, the generation of bioactive peptides and the accumulation of soluble species inside LDCV. This latter feature of Cgs accounts for the ability of vesicles to concentrate catecholamines and Ca(2+). Indeed, the low affinity and high capacity of Cgs to bind solutes at the low pH of the LDCV lumen seems to be behind the delay in the neurotransmitter exit towards the extracellular milieu after vesicle fusion. The availability of new mouse strains lacking Cgs in combination with the arrival of several techniques for the direct monitoring of exocytosis (like amperometry, patch-amperometry and intracellular electrochemistry), have helped advance our understanding of how these granins concentrate catecholamines and Ca(2+) in LDCV, and how they influence the kinetics of exocytosis. In this review, we will discuss the roles of Cgs A and B in maintaining the intravesicular environment of secretory vesicles and in exocytosis, bringing together the most recent findings from adrenal chromaffin cells.
Borges R, D¨ªaz-Vera J, Dom¨ªnguez N et al., J Neurochem. 2010 Jul;114(2):335-43. doi: 10.1111/j.1471-4159.2010.06786.x. Epub 2010 Apr 29.
|
|
|
L-009
|
|
|


