|
|
|
Relaxin-3 / Insulin-like Peptide 7
(INSL-7) |
A New Orexigenic Peptide
Stimulates Feeding |
Relaxin-3 (INSL-7) is a
recently discovered member of the insulin
superfamily. Relaxin-3 mRNA is expressed in the
nucleus incertus of the brain stem which has
projections to the hypothalamus. Relaxin-3 binds
with high affinity to the LGR7 receptor and to the
previously orphan G protein-coupled receptor
GPCR135. GPCR135 mRNA is expressed predominantly
in the CNS, particularly in the paraventricular
nucleus (PVN). The presence of relaxin-3 and these
receptors in the PVN led us to investigate the
effect of central administration of relaxin-3 on
food intake in male Wistar rats. The receptor
involved in mediating these effects was also
investigated. Intracerebroventricular (ICV)
injections of human relaxin-3 (H3) to satiated
rats significantly increased food intake 1 h
post-administration in the early light phase [0.96
+/- 0.16 g (vehicle) vs. 1.81 +/- 0.21 g (180 pmol
H3), P < 0.05] and the early dark phase [2.95
+/- 0.45 g (vehicle) vs. 4.39 +/- 0.39 g (180 pmol
H3), P < 0.05]. IntraPVN H3 administration
significantly increased 1 h food intake in
satiated rats in the early light phase [0.34 +/-
0.16 g (vehicle) vs. 1.23 +/- 0.30 g (18 pmol H3),
P < 0.05] and the early dark phase [4.43 +/-
0.32 g (vehicle) vs. 6.57 +/- 0.42 g (18 pmol H3),
P < 0.05]. Feeding behavior increased following
iPVN H3. Equimolar doses of human relaxin-2, which
binds the LGR7 receptor but not GPCR135, did not
increase feeding. Hypothalamic NPY, POMC or AgRP
mRNA expression did not change following acute ICV
H3. These results suggest a novel role for
relaxin-3 in appetite regulation.
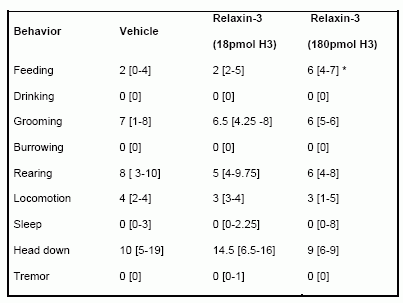
Effect of iPVN administration of H3 (18 pmol or 180 pmol) on behavior in the first hour following injection. Behavior was classified into one of nine categories. Each rat was observed for three 3 sec periods every 6 min and the behavior in each period scored as previously described (19). Behavioral data are expressed as median number of occurrences of behavior (interquartile ranges are expressed in square brackets), * = p < 0.05 vs vehicle.
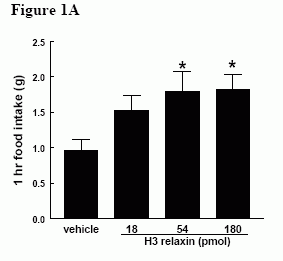 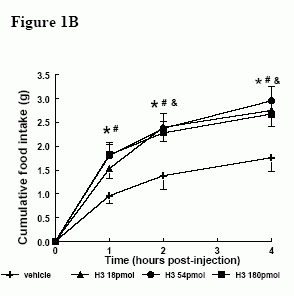 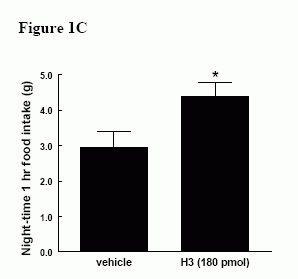
Effect of ICV administration of relaxin-3 in satiated male Wistar rats. A) Effect of H3 (18-180 pmol) on 1h food intake * = p < 0.05 vs vehicle in early light phase B) Effect of H3 (18-180 pmol) on cumulative food intake over 4h in early light phase. & = p < 0.05 at 18 pmol vs vehicle, * = p < 0.05 at 54 pmol vs vehicle, # = p < 0.05 at 180 pmol vs vehicle C) Effect of H3 (180 pmol) on 1h food intake in early dark phase, * = p < 0.05 vs vehicle.
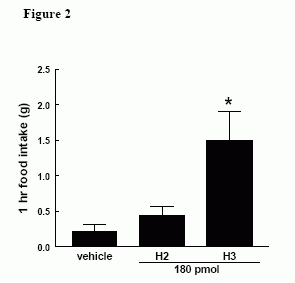
Effect of ICV administration of H3 (180 pmol) and H2 (180 pmol) on 1h food intake in early light phase in satiated male Wistar rats, * = p < 0.05 vs vehicle.
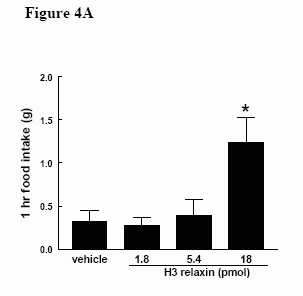 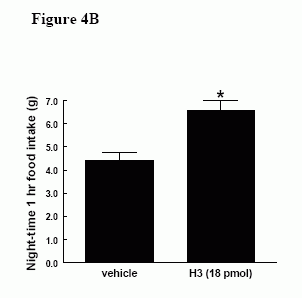
Effect of iPVN administration of H3 in male Wistar rats. A) Effect of H3 (1.8-18 pmol) on 1h food intake in early light phase B) Effect of H3 (18 pmol) on 1h food intake in early dark phase, * = p < 0.05 vs vehicle.
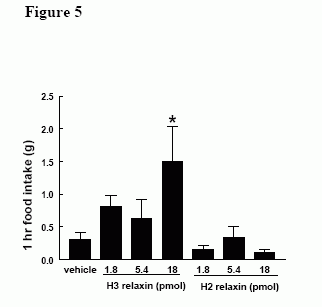
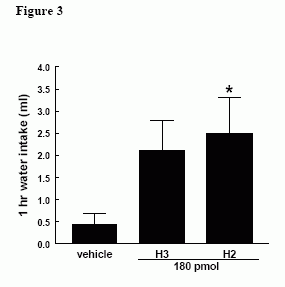
Effect of iPVN administration of equimolar doses of H3 (18 pmol) and H2 (18pmol) on 1h food intake in satiated male Wistar rats, * = p < 0.05 vs vehicle.
McGowan BM, et al. Endocrinology. 2005 Apr 21; [Epub ahead of print]
Liu C, et al. J. Biol. Chem., Vol. 278, 50765-50770, December 12, 2003
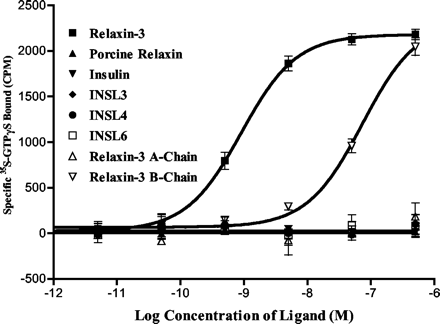
Relaxin-3 stimulates 35S-GTPS binding in GPCR142 expressing cells. Different peptides with various concentrations were added to the human GPCR142 expressing cell membranes to stimulate GTPS incorporation. The specific 35S-GTPS incorporation was obtained by subtracting counts without ligand from the counts with ligand.
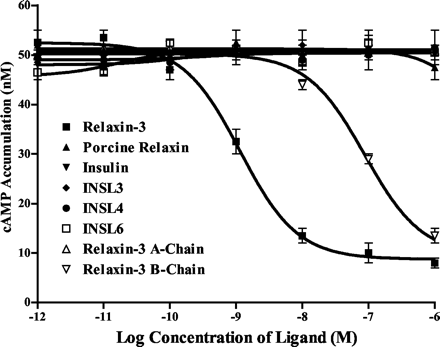
A, inhibition of forskolin-stimulated cAMP production by relaxin-3. Cells stably expressing GPCR142 were established in SK-N-MC cells. Different peptides with various concentrations were added to the cell cultures. Forskolin was then added to all samples at a final concentration of 5 µM. Forskolin-stimulated cAMP accumulation was measured using cAMP Flash Plates (PerkinElmer Life Sciences).
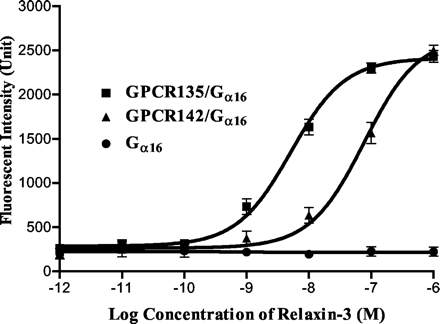
Relaxin-3 stimulates Ca2+ mobilization in HEK293 cells co-expressing GPCR142 and G16. HEK293 cells, either transfected with G16 alone (G16), or co-transfected by human GPCR142 and G16 (GPCR142/G16), were used for Ca2+ mobilization assays using different concentrations of relaxin-3 as the ligand. Ligand-stimulated intracellular Ca2+ mobilization was monitored by FLIPR. HEK293 cells co-transfected by G16 and GPCR135 (GPCR135/G16) were used in the same experiment for comparison.
Liu C, et al. J. Biol. Chem., Vol. 278, 50765-50770, December 12, 2003
We have recently identified the insulin-like peptide relaxin-3 (aka INSL7) as the endogenous ligand for an orphan G-protein-coupled receptor, GPCR135 (aka somatostatin- and angiotensin-like peptide receptor). Analysis of possible receptors related to GPCR135 revealed a single orphan receptor, GPCR142. Thus, we tested whether GPCR142 could also respond to relaxin-3 or related insulin-like molecules. Surprisingly, GPCR142 was activated by nanomolar concentrations of relaxin-3 but was completely unresponsive to all other known insulin-like peptides. We evaluated by reverse transcriptase-PCR the expression of GPCR142 mRNA in a variety of human tissues and found expression in brain, kidney, testis, thymus, placenta, prostate, salivary gland, thyroid, and colon. In an analysis of other species, we were able to find a full-length mouse homolog of GPCR142, but were unable to detect any complete GPCR142 transcripts in rat. With respect to intracellular signaling, GPCR142 is similar to GPCR135 in that it potently inhibits adenylate cyclase and stimulates 35S-GTPgammaS incorporation in response to relaxin-3. However, whereas GPCR135 signaling could be converted to calcium mobilization using a Gqi5 or Galpha16 G-proteins, GPCR142 was only capable of functioning in the presence of Galpha16. In the accompanying article (Liu, C., Eriste, E., Sutton, S., Chen, J., Roland, B., Kuei, C., Farmer, N., Jornvall, H., Sillard, R., and Lovenberg, T. W. (2003) J. Biol. Chem. 278, 50754-50764), we present the case that relaxin-3, which has previously been shown to bind to the relaxin receptor LGR7, is most likely the endogenous ligand for GPCR135. In this report, we show an additional receptor, GPCR142, which is also selectively activated by relaxin-3. However, the anatomical localization of GPCR142 suggests that GPCR142 may have different physiological functions.
Liu C, et al. J Biol Chem. 2003 Dec 12;278(50):50765-70. Epub 2003 Sep 30.
The omnipresent 6kDa polypeptide relaxin (RLX) is emerging as a multi-functional endocrine and paracrine factor, with a broad range of target tissues that includes the cardiovascular system. Humans and other higher primates have three RLX genes, designated H1, H2 and H3, of which H2 RLX is the major stored and circulating form. Rodents have only two RLX genes: relaxin-1 (equivalent to H2 RLX) and relaxin-3 (equivalent to H3 RLX). The recent cloning of the human RLX receptor (LGR7), a member of the leucine-rich repeat family of G-protein-coupled orphan receptors, and detection of LGR7 gene transcripts in the heart confirm this organ as a target for RLX (H2). However, evidence for production of the ligand within the cardiovascular system is limited, and few studies have clearly identified the physiological effects of RLX on cardiac function. To add to the controversy, serum concentrations and expression of RLX in the heart are elevated in chronic heart failure patients and animal models of cardiomyopathy, implying that RLX may only be a marker for pathological cardiovascular conditions, rather than normal physiology.
Samuel CS, Parry LJ, Summers RJ. Curr Opin Pharmacol 2003 Apr;3(2):152-8
Leucine-rich repeat-containing, G protein-coupled receptors (LGRs) represent a unique subgroup of G protein-coupled receptors with a large ectodomain. Recent studies demonstrated that relaxin activates two orphan LGRs, LGR7 and LGR8, whereas INSL3/Leydig insulin-like peptide specifically activates LGR8. Human relaxin 3 (H3 relaxin) was recently discovered as a novel ligand for relaxin receptors. Here, we demonstrated that H3 relaxin activates LGR7 but not LGR8. Taking advantage of the overlapping specificity of these three ligands for the two related LGRs, chimeric receptors were generated to elucidate the mechanism of ligand activation of LGR7. Chimeric receptor LGR7/8 with the ectodomain from LGR7 but the transmembrane region from LGR8 maintains responsiveness to relaxin but was less responsive to H3 relaxin based on ligand stimulation of cAMP production. The decreased ligand signaling was accompanied by decreases in the ability of H3 relaxin to compete for 33P-relaxin binding to the chimeric receptor. However, replacement of the exoloop 2, but not exoloop 1 or 3, of LGR7 to the chimeric LGR7/8, restored ligand binding and receptor-mediated cAMP production. These results suggested that activation of LGR7 by H3 relaxin involves specific binding of the ligand to both the ectodomain and the exoloop 2, thus providing a model with which to understand the molecular basis of ligand signaling for this unique subgroup of G protein-coupled receptors.
Sudo S. et al. J. Biol. Chem, Feb 2003; 278: 7855 - 7862.
Relaxin is an insulin-like peptide consisting of two separate chains (A and B) joined by two inter- and one intrachain disulfide bonds. Binding to its receptor requires an Arg-X-X-X-Arg-X-X-Ile motif in the B-chain. A related member of the insulin superfamily, INSL3, has a tertiary structure that is predicted to be similar to relaxin. It also possesses an Arg-X-X-X-Arg motif within its B-chain, although this is displaced by four amino acids towards the C-terminus from the corresponding position within relaxin. We have previously shown that synthetic INSL3 itself does not display relaxin-like activity although analogue (Analogue A) with an introduced arginine residue in the B-chain giving it an Arg cassette in the exact relaxin position does possess weak activity. In order to identify further the structural features that impart relaxin function, solid phase peptide synthesis was used to prepare three additional analogues for bioassay. Each of these contained point substitutions within the arginine cassette. Analogue D contained the full human relaxin binding cassette, Analogue G consisted of the native INSL3 sequence containing an Arg to Ala substitution, and Analogue E was a further modification of Analogue A, with the same substitution. Each analogue was fully chemically characterized by a number of criteria. Detailed circular dichrosim spectroscopy analyses showed that the changes caused little alteration of secondary structure and, hence, overall conformation. However, each analogue displayed only weak relaxin-like activity. These results indicate that while the arginine cassette is vital for relaxin-like activity, there are additional, as yet unidentified structural requirements for relaxin binding.
Claasz AA, Bond CP, Bathgate RA, Otvos L, Dawson NF, Summers RJ, Tregear GW, Wade JD. Eur J Biochem 2002 Dec; 269(24):6287-93
Relaxin is a peptide hormone with known actions associated with female reproductive physiology, but it has also been identified in the brain. Only one relaxin gene had been characterized in rodents until recently when a novel human relaxin gene, human gene-3 (H3) and its mouse equivalent (M3) were identified. The current study reports the identification of a rat homologue, rat gene-3 (R3) relaxin that is highly expressed in a discrete region of the adult brain. The full R3 relaxin cDNA was generated using RT-PCR and 3' and 5' RACE protocols. The derived amino acid sequence of R3 relaxin retains all the characteristic features of a relaxin peptide and has a high degree of homology with H3 and M3 relaxin. The distribution of R3 relaxin mRNA in adult rat brain was determined and highly abundant expression was only detected in neurons of the ventromedial dorsal tegmental nucleus (vmDTg) in the pons, whereas all other brain areas were unlabelled or contained much lower mRNA levels. Relaxin binding sites and relaxin immunoreactivity were also detected in the vmDTg. These together with earlier findings provide strong evidence for a role(s) for multiple relaxin peptides as neurotransmitters and/or modulators in the rat CNS.
Burazin TC, Bathgate RA, Macris M, Layfield S, Gundlach AL, Tregear GW. J Neurochem 2002 Sep;82(6):1553-7
The relaxin-like factor (RLF), which is the product of the insulin-like factor 3 (INSL3) gene, is a new circulating peptide hormone of the relaxin-insulin family. In male mammals, it is a major secretory product of the testicular Leydig cells, where it appears to be expressed constitutively but in a differentiation-dependent manner. In the adult testis, RLF expression is a good marker for fully differentiated adult-type Leydig cells, but it is only weakly expressed in prepubertal immature Leydig cells or in Leydig cells that have become hypertrophic or transformed. It is also an important product of the fetal Leydig cell population, where it has been demonstrated using knockout mice to be responsible for the second phase of testicular descent acting on the gubernaculum. INSL3 knockout mice are cryptorchid, and in estrogen-induced cryptorchidism, RLF levels in the testis are significantly reduced. RLF is also made in female tissues, particularly in the follicular theca cells of small antral follicles and in the corpus luteum of the cycle and pregnancy. The ruminant ovary has a very high level of RLF expression, and analysis of primary cultures of ovarian theca-lutein cells indicated that, as in the testis, expression is probably constitutive but differentiation dependent. Female INSL3 knockout mice have altered estrous cycles, where RLF may be involved in follicle selection, an idea strongly supported by observations on bovine secondary follicles. Recently, a novel 7-transmembrane domain receptor (LGR8 or Great) has been tentatively identified as the RLF receptor, and its deletion in mice leads also to cryptorchidism.
Ivell R, Bathgate RA. Biol Reprod 2002 Sep;67(3):699-705
Several orphan G protein-coupled receptors homologous to gonadotropin and thyrotropin receptors have recently been identified and named as LGR4-8. INSL3, also known as Leydig insulin-like peptide or relaxin-like factor, is a relaxin family member expressed in testis Leydig cells and ovarian theca and luteal cells. Male mice mutant for INSL3 exhibit cryptorchidism or defects in testis descent due to abnormal gubernaculum development whereas overexpression of INSL3 induces ovary descent in transgenic females. Because transgenic mice missing the LGR8 gene are also cryptorchid, INSL3 was tested as the ligand for LGR8. Here, we show that treatment with INSL3 stimulated cAMP production in cells expressing recombinant LGR8 but not LGR7. In addition, interactions between INSL3 and LGR8 were demonstrated following ligand receptor cross-linking. Northern blot analysis indicated that the LGR8 transcripts are expressed in gubernaculum whereas treatment of cultured gubernacular cells with INSL3 stimulated cAMP production and thymidine incorporation. The present study identified the ligand for an orphan G protein-coupled receptor based on common phenotypes of ligand and receptor null mice. Demonstration of INSL3 as the ligand for LGR8 facilitates understanding of the mechanism of testis descent and allows studies on the role of INSL3 in gonadal and other physiological processes.
Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ. J Biol Chem 2002 Aug 30;277(35):31283-6
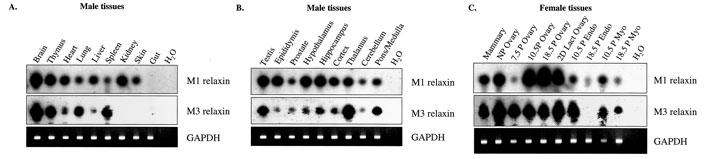
| 
| |
Southern blot analysis of M1 and M3 relaxin gene expression in the mouse. Southern blots were conducted on RT-PCR products (from various male and female mouse tissues) using specific internal oligonucleotide primers to M1 and M3 relaxin cDNA, respectively. UV photographs of GAPDH PCR products are shown as controls for quality, and equivalent loading, of the cDNA. Water (H2O) replaced cDNA in negative control reactions for each PCR. Relaxin mRNA expression was determined in a number of non-reproductive male tissues (A), in reproductive tissues and specific brain regions of the male (B), and in female reproductive tissues at different stages of pregnancy (C). |
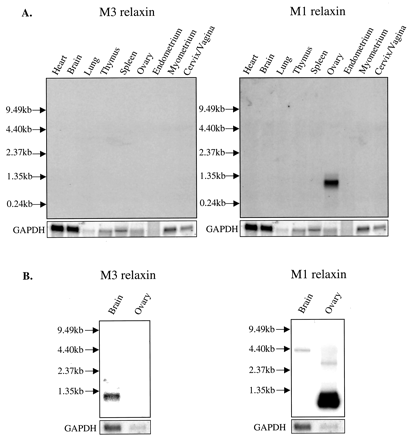
Northern blot analysis of M1 and M3 relaxin mRNA expression in the mouse. Total RNA (5-25 µg) from male non-reproductive and female pregnant reproductive mouse tissues (A) were hybridized separately with 32P-labeled single-stranded M3 relaxin, M1 relaxin, and GAPDH probes. The RNA from the female reproductive tissues was pooled from day 7.5, 10.5, and 18.5 of pregnancy. B, poly(A) RNA from male brain (15 µg; purified from 200 µg of total RNA) and total RNA (5 µg) from the pregnant (day 18.5) ovary (B) were also probed with the M3 relaxin, M1 relaxin, and GAPDH probes. The positions of a 0.24-9.5-kb RNA ladder are indicated. Note the specific signals for M1 relaxin (~1 kb) in the ovary and for M3 relaxin (~1.2 kb) in brain poly(A) RNA.
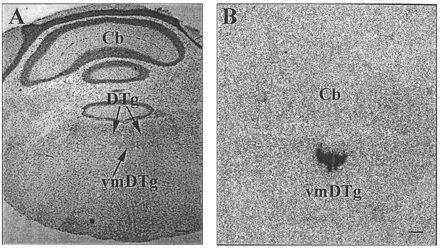
In situ hybridization histochemical localization of M3 relaxin mRNA in mouse brain. A near adjacent thionin-stained section (A) reveals the distribution of M3 relaxin mRNA (B) to be localized to the pars ventromedialis of the dorsal tegmental nucleus (vmDTg), situated ventral to the dorsal tegmental nucleus (DTg), within the pons/medulla. An x-ray film image of M3 relaxin mRNA enriched in the pars ventromedialis of the dorsal tegmental nucleus of the mouse pons/medulla (B), but not in the cerebellum (Cb). Scale bar: A, 1 mm; B, 0.5 mm.
J. Biol. Chem., Vol. 277, Issue 2, 1148-1157, January 11, 2002
|
|
|
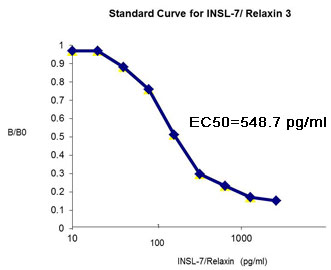
Relaxin;Relaxin-2;INSL3;INSL4;INSL5;INSL6;INSL7_C_peptide
%INSL 7%
|
|
|


