|
|
|
Insulin-like
5 Peptide (INSL5) |
A Circulated Hormone that Potentiates Insulin/GLP-1 Secretion and Plays a Role in Glucose Homeostasis and Obesity |
Insulin-like peptide 5 (INSL5), a member of the insulin/relaxin superfamily, can activate the G-protein coupled receptor RXFP4, but its precise biological functions are largely unknown. Recent studies suggest that the INSL5/RXFP4 is involved in the control of food intake and glucose homeostasis. We report here that RXFP4 is present in the mouse insulinoma cell line MIN6 and INSL5 augments glucose-stimulated insulin secretion (GSIS) both in vitro and in vivo. RXFP4 is also expressed in the mouse intestinal L cell line GLUTag and INSL5 is capable of potentiating glucose-dependent glucagon-like peptide-1 (GLP-1) secretion in GLUtag cells. We propose that the insulinotropic effect of INSL5 is probably mediated through stimulation of insulin/GLP-1 secretion and INSL5 may be a potential therapeutic target for type 2 diabetes.
Luo X, Li T, Zhu Y, Dai YB et al., Biochem J. 2014 Dec 17. [Epub ahead of print]
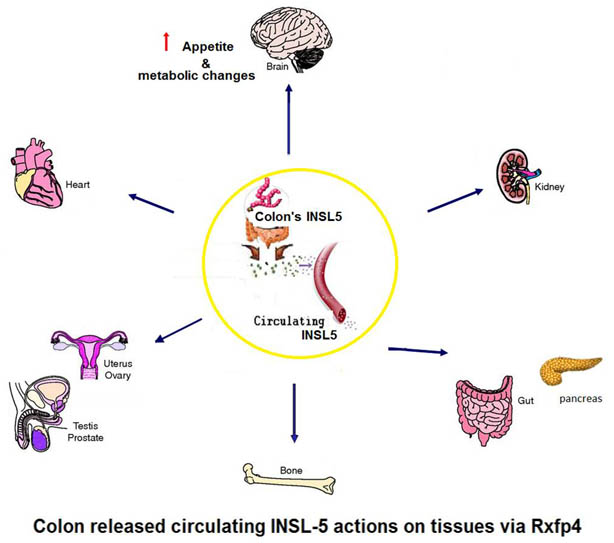
The gut endocrine system is emerging as a central player in the control of appetite and glucose homeostasis, and as a rich source of peptides with therapeutic potential in the field of diabetes and obesity. In this study we have explored the physiology of insulin-like peptide 5 (Insl5), which we identified as a product of colonic enteroendocrine L-cells, better known for their secretion of glucagon-like peptide-1 and peptideYY. i.p. Insl5increased food intake in wild-type mice but not mice lacking the cognate receptor Rxfp4. Plasma Insl5 levels were elevated by fasting or prolonged calorie restriction, and declined with feeding. We conclude that Insl5 is an orexigenic hormone released from colonic L-cells, which promotes appetite during conditions of energy deprivation.
Grosse J, Heffron H, Burling K et al, Proc Natl Acad Sci U S A. 2014 Jul 15. pii: 201411413. [Epub ahead of print]
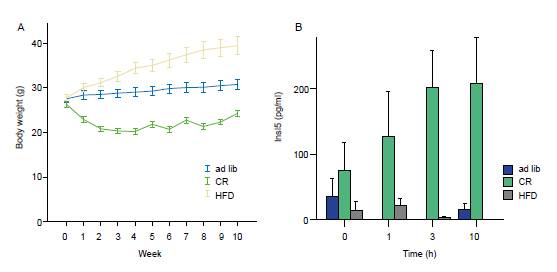
Changes of body weight and plasma INSL 5 levels after dietary intervention. (A and B) Effect of long-term dietary alterations on Insl5 levels. C57BL/6J mice were fed with standard chow ad libitum (ad lib) or restricted to 60% of the former (calorie restriction; CR), or with a high-fat diet (HFD, 45% fat) for 10 wk. (A) Body weight changed as expected in the cohorts, represented as mean ± 95%CI. (B) After an overnight fast, terminal blood samples were taken before or at the indicated time after refeeding. Insl5 levels were higher in the CR cohort. Plasma Insl5 levels were determined. Mouse Insl5 was measured using a RIA kit from Phoenix Pharmaceuticals. The specificity of the assay was confirmed by detection of synthetic Insl5 (Phoenix Pharmaceuticals)
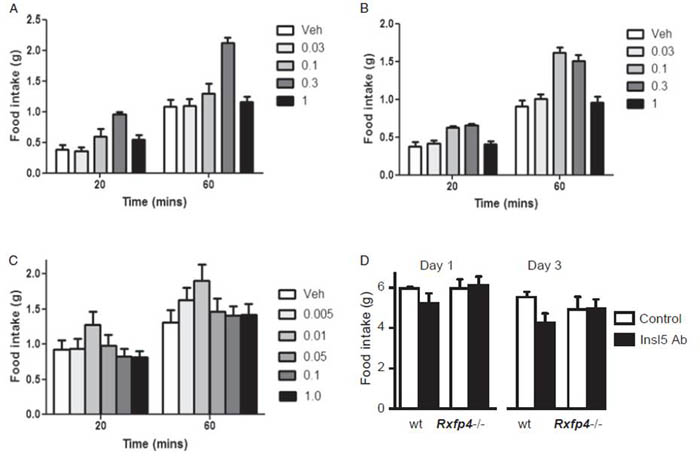
Effect of Insl5 on food intake in mice. (A) Animals were adapted to a palatable wet mash meal (made from chow diet) at a fixed time of day. Commercial Insl5 (Phoenix Pharmaceuticals), at the doses indicated, was injected and food intake was monitored. (D) The effect of AB-mediated neutralization of endogenous Insl5 is abrogated in receptor-deficient mice: Wild-type or Rxfp4−/− mice were treated as described in Fig. 4C with 50 μg of anti-Insl5 antibody (black bars) or rabbit normal serum (white bars). Insl5 Ab resulted in a reduced food intake in wild-type but not Rxfp4−/− mice (P < 0.001, GLM for repeated measures, n = 5; Tukey HSD post hoc: P < 0.001 for the wild-type group treated with anti-Insl5 Ab vs. all other groups). Data are presented as mean + 95% CI.
Insl5 for i.p. administration was purchased from Phoenix Pharmaceuticals. Neutralization of endogenous Insl5 was performed using polyclonal anti-Insl5 Ab ( Phoenix Pharmaceuticals).
Figure from supporting information of Proc Natl Acad Sci U S A. 2014 Jul 15.
Insulin-like peptide 5 (INSL5) is an insulin/relaxin superfamily peptide involved in the regulation of glucose homeostasis by activating its receptor RXFP4, which can also be activated by relaxin-3 in vitro. To determine the interaction mechanism of INSL5 with its receptor RXFP4, we studied their electrostatic interactions using a charge-exchange mutagenesis approach. First, we identified three negatively charged extracellular residues (Glu100, Asp104 and Glu182) in human RXFP4 that were important for receptor activation by wild-type INSL5. Second, we demonstrated that two positively charged B-chain Arg residues (B13Arg and B23Arg) in human INSL5 were involved in receptor binding and activation. Third, we proposed probable electrostatic interactions between INSL5 and RXFP4: the B-chain central B13Arg of INSL5 interacts with both Asp104 and Glu182 of RXFP4, meanwhile the B-chain C-terminal B23Arg of INSL5 interacts with both Glu100 and Asp104 of RXFP4. The present electrostatic interactions between INSL5 and RXFP4 were similar to our previously identified interactions between relaxin-3 and RXFP4, but had subtle differences that might be caused by the different B-chain C-terminal conformations of relaxin-3 and INSL5 because a dipeptide exchange at the B-chain C-terminus significantly decreased the activity of INSL5 and relaxin-3 to receptor RXFP4.
Wang XY, Guo YQ, Shao XX et al., Arch Biochem Biophys. 2014 Sep 15;558:127-32. doi: 10.1016/j.abb.2014.07.010. Epub 2014 Jul 18.
The gut endocrine system is emerging as a central player in the control of appetite and glucose homeostasis, and as a rich source of peptides with therapeutic potential in the field of diabetes and obesity. In this study we have explored the physiology of insulin-like peptide 5 (Insl5), which we identified as a product of colonic enteroendocrine L-cells, better known for their secretion of glucagon-like peptide-1 and peptideYY. i.p. Insl5increased food intake in wild-type mice but not mice lacking the cognate receptor Rxfp4. Plasma Insl5 levels were elevated by fasting or prolonged calorie restriction, and declined with feeding. We conclude that Insl5 is an orexigenic hormone released from colonic L-cells, which promotes appetite during conditions of energy deprivation.
Grosse J, Heffron H, Burling K et al., Proc Natl Acad Sci U S A. 2014 Jul 29;111(30):11133-8. doi: 10.1073/pnas.1411413111. Epub 2014 Jul 15.
Insulin-like peptide 5 (INSL5) is a member of the insulin superfamily, and is a potent agonist for RXFP4. We have shown that INSL5 is expressed in enteroendocrine cells (EECs) along the colorectum with a gradient increase toward the rectum. RXFP4 is ubiquitously expressed along the digestive tract. INSL5-positive EECs have little immunoreactivity to chromogranin A (CgA) and might be a unique marker of colorectal EECs. CgA-positive EECs were distributed normally along the colorectum in INSL5 null mice, suggesting that INSL5 is not required for the development of CgA-positive EECs. Exogenous INSL5 did not affect the proliferation of human colon cancer cell lines, and chemically-induced colitis in INSL5 null mice did not show any significant changes in inflammation or mucosal healing compared to wild-type mice. In contrast, all of the rectal neuroendocrine tumors examined co-expressed INSL5 and RXFP4. INSL5 may be a unique marker of colorectal EECs, and INSL5-RXFP4 signaling might play a role in an autocrine/paracrine fashion in the colorectal epithelium and rectal neuroendocrine tumors.
Mashima H et al, Biochem Biophys Res Commun. 2013 Mar 22;432(4):586-92. doi: 10.1016/j.bbrc.2013.02.042. Epub 2013 Feb 22.
Insulin-like factor 5 (INSL5), a member of the insulin superfamily, is expressed in the colorectum and hypothalamus. To facilitate studies into the role of INSL5, we generated Insl5(-/-) mice by gene targeting. Insl5(-/-) mice were born in the expected Mendelian ratio, reached normal body weight, but displayed impaired male and female fertility that are due to marked reduction in sperm motility and irregular length of the estrous cycle. Furthermore, Insl5(-/-) mice showed impairment in glucose homeostasis with characteristic elevation of serum glucose levels at an advanced age. Glucose and insulin tolerance tests revealed that the increased blood glucose in Insl5(-/-) mice was due to glucose intolerance resulting from reduced insulin secretion. Morphometric and immunohistological analyses revealed that the Insl5(-/-) mice had markedly reduced average islets area and β-cell numbers. Furthermore, immunohistochemistry showed the expression of INSL5 in enteroendocrine cells in the colorectal epithelium and the presence of its putative receptor relaxin family peptide receptor 4 in pancreatic islet cells. These results suggest the potential role of INSL5 signaling in the regulation of insulin secretion and β-cell homeostasis.
Burnicka-Turek O et al., Endocrinology. 2012 Oct;153(10):4655-65. Epub 2012 Jul 20.
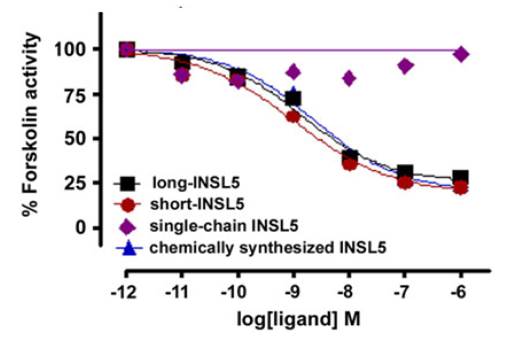
Insulin-like peptide 5 (INSL5) is a recently identified insulin superfamily member. Although it binds to and activates the G-protein coupled receptor, RXFP4, its precise biological function remains unknown. To help determine its function, significant quantities of INSL5 are required. In the present work, three single-chain INSL5 precursors were designed, two of which were successfully expressed in E. coli cells. The expressed precursors were solubilized from inclusion bodies, purified almost to homogeneity by immobilized metal-ion affinity chromatography, and then refolded in vitro. One precursor could be converted to two-chain human INSL5 bearing an extended N-terminus of the A-chain (designated long-INSL5) by sequential Lys-C endoproteinase and carboxypeptidase B treatment. The 6 residue A-chain N-terminal extension of long-INSL5 was subsequently removed by Aeromonas aminopeptidase to yield native INSL5 that was designated short-INSL5. Circular dichroism spectroscopic analysis and peptide mapping showed that the recombinant INSL5s adopted an insulin-like conformation and possessed the expected characteristic insulin-like disulfide linkages. Activity assay showed that both long- and short-INSL5 had full RXFP4 receptor activity compared with chemically synthesized human INSL5. This suggested that extension of the N-terminus of the A-chain of long-INSL5 did not adversely impact upon the binding to or activation of the RXFP4 receptor. However, the single-chain INSL5 precursor was inactive which indicated that a free C-terminus of the B-chain is critical for the activity ofINSL5. Our present work thus provides an efficient approach for preparation of INSL5 and its analogs through recombinant expression in E. coli cells.
Luo X, Bathgate RA, Zhang WJ et al., Amino Acids. 2010 Nov;39(5):1343-52. doi: 10.1007/s00726-010-0586-3. Epub 2010 Apr 11.
The receptors for members of the relaxin peptide family have only recently been discovered and are G-protein-coupled receptors (GPCRs). Relaxin and insulin-like peptide 3 (INSL3) interact with the leucine-rich-repeat-containing GPCRs (LGRs) LGR7 and LGR8, respectively. These receptors show closest similarity to the glycoprotein hormone receptors and contain large ectodomains with 10 leucine-rich repeats (LRRs) but are unique members of the LGR family (class C) as they have an LDL class A (LDLa) module at their N-terminus. In contrast, relaxin-3 and INSL5 interact with another class of type I GPCRs which lack a large ectodomain, the peptide receptors GPCR135 and GPCR142, respectively. These receptors are now classified as relaxin family peptide (RXFP) receptors, RXFP1 (LGR7), RXFP2 (LGR8), RXFP3 (GPCR135) and RXFP4 (GPCR142). This review outlines the identification of the peptides and receptors, their expression profiles and physiological roles and the functional interactions of the peptides with their unique receptors.
Kong RC, Shilling PJ, Lobb DK et al., Mol Cell Endocrinol. 2010 May 14;320(1-2):1-15. doi: 10.1016/j.mce.2010.02.003. Epub 2010 Feb 6.
Insulin-like peptide 5 (INSL5) was first identified through searches of the expressed sequence tags (EST) databases. Primary sequence analysis showed it to be a prepropeptide that was predicted to be processed in vivo to yield a two-chain sequence (A and B) that contained the insulin-like disulfide cross-links. The high affinity interaction between INSL5 and the receptor RXFP4 (GPCR142) coupled with their apparent coevolution and partially overlapping tissue expression patterns strongly suggest that INSL5 is an endogenous ligand for RXFP4. Given that the primary function of the INSL5-RXFP4 pair remains unknown, an effective means of producing sufficient quantities of this peptide and its analogues is needed to systematically investigate its structural and biological properties. A combination of solid-phase peptide synthesis methods together with regioselective disulfide bond formation were used to obtain INSL5. Both chains were unusually resistant to standard synthesis protocols and required highly optimized conditions for their acquisition. In particular, the use of a strong tertiary amidine, DBU, as N(alpha)-deprotection base was required for the successful assembly of the B chain; this highlights the need to consider incomplete deprotection rather than acylation as a cause of failed synthesis. Following sequential disulfide bond formation and chain combination, the resulting synthetic INSL5, which was obtained in good overall yield, was shown to possess a similar secondary structure to human relaxin-3 (H3 relaxin). The peptide was able to inhibit cAMP activity in SK-N-MC cells that expressed the human RXFP4 receptor with a similar activity to H3 relaxin. In contrast, it had no activity on the human RXFP3 receptor. Synthetic INSL5 demonstrates equivalent activity to the recombinant-derived peptide, and will be an important tool for the determination of its biological function.
Akhter Hossain M, Bathgate RA, Kong CK, et al. Chembiochem. 2008 Jul 21;9(11):1816-22. doi: 10.1002/cbic.200800113.

|
|
| Peptide |
Cross-reactivity |
| INSL 5 (Human)
|
100% |
| Insulin (Human) |
0% |
| INSL 3 (Human)
|
0% |
| INSL 4 (Human)
|
0% |
| INSL 6 (Human)
|
0% |
| INSL 7 (Relaxin 3) (Human)
|
0% |
| Relaxin 2 (Human)
|
0% |
| Vesiculin (Human) |
0% |
| Ghrelin (Human) |
0% |
| GLP-1 (Human) |
0% |
| PYY (3-36) (Human)
|
0% |
|
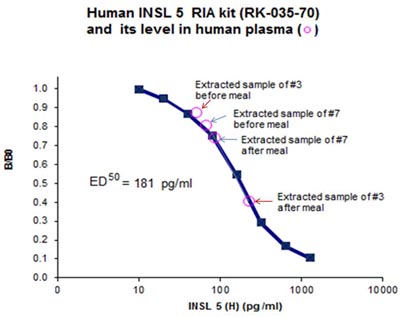
Minimum detection level: 80 pg/ml
Assay Range: 20 - 2560 pg/ml
|
|
| Peptide |
Cross-reactivity |
| INSL 5 (Human)
|
100% |
| Insulin (Human) |
0% |
| INSL 3 (Human) |
0% |
| INSL 6 (Mouse)
|
0% |
| INSL 7 (Relaxin 3) (Human)
|
0% |
| Relaxin 2 (Human)
|
0% |
|
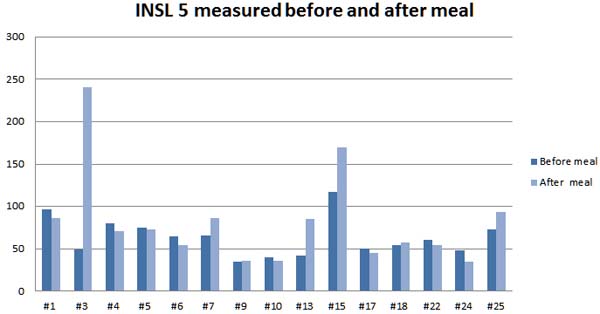
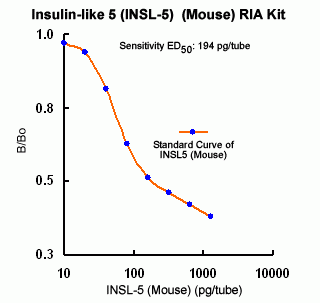
Minimum detection level: 300 pg/ml
Assay Range: 100-12800 pg/ml
|
|
| Peptide |
Cross-reactivity |
| INSL 5 (Mouse)
|
100% |
| INSL 5 (Human)
|
0.13 % |
| INSL 3 (Mouse)
|
0% |
| INSL 3 (Human)
|
0% |
| INSL 4 (Human)
|
0% |
| INSL 6 (Mouse)
|
0% |
| INSL 7 (Relaxin 3) (Human)
|
0% |
| Relaxin 2 (Human)
|
0% |
| Insulin (Human)
|
0% |
| INSL 7 C-peptide (Human)
|
0% |
|
Linear Range: 0.33-4.53 ng/ml
Assay Range: 0.1-100 ng/ml
|
|
| Peptide |
Cross-reactivity |
INSL 5 C-Peptide, Prepro(49-106) (Human)
|
100% |
INSL 2 C-Peptide tide (Human) |
0% |
| INSL 3 C-Peptide (Human) |
0% |
| INSL 4 C-Peptide (Human) |
0% |
| INSL 6 C-Peptide (Human) |
0% |
INSL 7 C-Peptide (Human) |
0% |
| Insulin (Human) |
0% |
|

Linear Range: 598.2-4250 pg/ml
Assay Range: 100-12800 pg/ml
|
|
| Peptide |
Cross-reactivity |
INSL 5 C-Peptide, Prepro(49-106) (Human) |
100% |
INSL 2 C-Peptide tide (Human) |
0% |
INSL 3 C-Peptide (Human) |
0% |
| INSL 4 C-Peptide (Human) |
0% |
| INSL 6 C-Peptide (Human) |
0% |
| INSL 7 C-Peptide (Human) |
0% |
Insulin (Human) |
0% |
|


Sequence comparison of human INSL5 (boxed) to INSL5 from other species (upper part), and to the remaining nine members of the human insulin/relaxin family (lower part)
Linda M. Haugaard-jönsson, Mohammed Akhter Hossain and others. Biochemical Journal (2009) 419, 619-627
Tips: See More Research Abstracts, Antibody Stainings, Immunoassay Kits Curves and Sequences by clicking the tabs on the top.
|
|
|
Relaxin;Relaxin-2;INSL3;INSL4;INSL6;INSL7;INSL5_C_Peptide
%INSL 5%
|
|
|


