|
|
|
Relaxin & Relaxin-Related
Peptides |
Insulin-like protein (INSL3, INSL4, INSL5, INSL6 & INSL7/H3
Relaxin) |
Insulin-like protein (INSL3, INSL4, INSL5, INSL6 & INSL7/H3
Relaxin) belongs to the insulin-like hormone superfamily, which
encompasses insulin, relaxin, and insulin-like growth factors I
(IGF1) and II (IGF2). Insulin gene superfamily hormones regulate
cell growth, metabolism, and tissue-specific functions.
Relaxin Receptor | Alternative names |
Ligand |
RXFP1 | LGR7 |
Relaxin1, Relaxin2, Relaxin3 |
RXFP2 | LGR8 |
Relaxin1, Relaxin2, INSL3 |
RXFP3 |
GPCR135, SALPR, RLN3R1 | Relaxin3 |
RXFP4 |
GPCR142, GPR100, RLN3R2 | INSL5, Relaxin 3 |
LGR7 Relaxin-1;Relaxin-2;INSL3;INSL4;INSL5;INSL6;INSL7 |
Insulin-like protein (INSL3, INSL4, INSL5, INSL6 & INSL7/H3
Relaxin) belongs to the insulin-like hormone superfamily, which
encompasses insulin, relaxin, and insulin-like growth factors
I (IGF1) and II (IGF2). Insulin gene superfamily hormones regulate
cell growth, metabolism, and tissue-specific functions. Members
of this family are characterized by a signal peptide, a B chain,
a connecting C chain, and an A chain. A preliminary in-house
study performed at Phoenix Pharmaceuticals, Inc. found gender
specific applications for INSL3. Results from analysis using
INSL3 RIA Kit (RK-035-27) showed the levels of circulating INSL3
in male samples were approximately ten times greater than that
found in female samples.
April 18, 2003 Phoenix Pharmaceuticals,
Inc. Belmont, CA
We have recently identified the insulin-like peptide relaxin-3
(aka INSL7) as the endogenous ligand for an orphan G-protein-coupled
receptor, GPCR135 (aka somatostatin- and angiotensin-like peptide
receptor). Analysis of possible receptors related to GPCR135
revealed a single orphan receptor, GPCR142. Thus, we tested
whether GPCR142 could also respond to relaxin-3 or related insulin-like
molecules. Surprisingly, GPCR142 was activated by nanomolar
concentrations of relaxin-3 but was completely unresponsive
to all other known insulin-like peptides. We evaluated by reverse
transcriptase-PCR the expression of GPCR142 mRNA in a variety
of human tissues and found expression in brain, kidney, testis,
thymus, placenta, prostate, salivary gland, thyroid, and colon.
In an analysis of other species, we were able to find a full-length
mouse homolog of GPCR142, but were unable to detect any complete
GPCR142 transcripts in rat. With respect to intracellular signaling,
GPCR142 is similar to GPCR135 in that it potently inhibits adenylate
cyclase and stimulates 35S-GTPgammaS incorporation in response
to relaxin-3. However, whereas GPCR135 signaling could be converted
to calcium mobilization using a Gqi5 or Galpha16 G-proteins,
GPCR142 was only capable of functioning in the presence of Galpha16.
In the accompanying article (Liu, C., Eriste, E., Sutton, S.,
Chen, J., Roland, B., Kuei, C., Farmer, N., Jornvall, H., Sillard,
R., and Lovenberg, T. W. (2003) J. Biol. Chem. 278, 50754-50764),
we present the case that relaxin-3, which has previously been
shown to bind to the relaxin receptor LGR7, is most likely the
endogenous ligand for GPCR135. In this report, we show an additional
receptor, GPCR142, which is also selectively activated by relaxin-3.
However, the anatomical localization of GPCR142 suggests that
GPCR142 may have different physiological functions.
Liu C, et al. J Biol Chem. 2003 Dec 12;278(50):50765-70. Epub
2003 Sep 30.
The omnipresent 6kDa polypeptide relaxin (RLX) is emerging as
a multi-functional endocrine and paracrine factor, with a broad
range of target tissues that includes the cardiovascular system.
Humans and other higher primates have three RLX genes, designated
H1, H2 and H3, of which H2 RLX is the major stored and circulating
form. Rodents have only two RLX genes: relaxin-1 (equivalent
to H2 RLX) and relaxin-3 (equivalent to H3 RLX). The recent
cloning of the human RLX receptor (LGR7), a member of the leucine-rich
repeat family of G-protein-coupled orphan receptors, and detection
of LGR7 gene transcripts in the heart confirm this organ as
a target for RLX (H2). However, evidence for production of the
ligand within the cardiovascular system is limited, and few
studies have clearly identified the physiological effects of
RLX on cardiac function. To add to the controversy, serum concentrations
and expression of RLX in the heart are elevated in chronic heart
failure patients and animal models of cardiomyopathy, implying
that RLX may only be a marker for pathological cardiovascular
conditions, rather than normal physiology.
Samuel CS, Parry LJ, Summers RJ. Curr
Opin Pharmacol 2003 Apr;3(2):152-8
The insulin-like hormone INSL-3, also named relaxin-like factor
(RLF) or Leydig-derived insulin-like peptide (LEY-IL), is expressed
in various reproductive tissues and is regarded a marker of
differentiation in human testicular Leydig cells. Recently,
we have identified differential expression of human INSL-3 in
neoplastic Leydig cells and mammary epithelial cells suggesting
an involvement of INSL-3 in tumor biology. Here we have investigated
the expression of INSL-3 in human thyroid carcinoma cell lines
and in the human thyroid gland which has been shown to express
transcripts for the G protein coupled INSL-3 receptor LGR8.
When we determined the expression of INSL-3 in eight human thyroid
carcinoma cell lines, a novel INSL-3 splice variant containing
a 95 bp out-of-frame insertion at the beginning of exon II of
the INSL-3 gene was discovered. Treatment of the human anaplastic
thyroid carcinoma cell line 8505C with diethylstilbestrol (DES)
caused a significant dose-dependent transcriptional down-regulation
of INSL-3 and a marked up-regulation of LGR8. Employing in situ
hybridization to detect INSL-3 transcripts and specific rabbit
antisera against the INSL-3 proteins, both INSL-3 isoforms were
detected in patients with Graves' disease (n=10), follicular
carcinomas (FTC; n=12), papillary carcinomas (PTC; n=9) and
undifferentiated anaplastic carcinomas (UTC; n=15). By contrast,
thyrocytes of all 15 benign goiter tissues studied were devoid
of both INSL-3 isoforms, mRNA and protein. Our data indicate
that INSL-3 hormone is up-regulated in hyperplastic and neoplastic
human thyrocytes suggesting that the INSL-3 isoforms may serve
as additional markers for hyperplastic and neoplastic human
thyrocytes. In the anaplastic thyroid carcinoma cell line 8505C,
the regulation of both INSL-3 and LGR8 by estrogen may be the
first indication of a novel hormonally responsive, auto-/paracrine
INSL-3 LGR8 ligand receptor system active in human thyroid carcinoma
cells.
Hombach-Klonisch S, et al. Int J Oncol
2003 May;22(5):993-1001
Leucine-rich repeat-containing, G protein-coupled receptors
(LGRs) represent a unique subgroup of G protein-coupled receptors
with a large ectodomain. Recent studies demonstrated that relaxin
activates two orphan LGRs, LGR7 and LGR8, whereas INSL3/Leydig
insulin-like peptide specifically activates LGR8. Human relaxin
3 (H3 relaxin) was recently discovered as a novel ligand for
relaxin receptors. Here, we demonstrated that H3 relaxin activates
LGR7 but not LGR8. Taking advantage of the overlapping specificity
of these three ligands for the two related LGRs, chimeric receptors
were generated to elucidate the mechanism of ligand activation
of LGR7. Chimeric receptor LGR7/8 with the ectodomain from LGR7
but the transmembrane region from LGR8 maintains responsiveness
to relaxin but was less responsive to H3 relaxin based on ligand
stimulation of cAMP production. The decreased ligand signaling
was accompanied by decreases in the ability of H3 relaxin to
compete for 33P-relaxin binding to the chimeric receptor. However,
replacement of the exoloop 2, but not exoloop 1 or 3, of LGR7
to the chimeric LGR7/8, restored ligand binding and receptor-mediated
cAMP production. These results suggested that activation of
LGR7 by H3 relaxin involves specific binding of the ligand to
both the ectodomain and the exoloop 2, thus providing a model
with which to understand the molecular basis of ligand signaling
for this unique subgroup of G protein-coupled receptors.
Sudo S. et al. J. Biol. Chem, Feb 2003;
278: 7855 - 7862.
Relaxin is a peptide hormone with known actions associated with
female reproductive physiology, but it has also been identified
in the brain. Only one relaxin gene had been characterized in
rodents until recently when a novel human relaxin gene, human
gene-3 (H3) and its mouse equivalent (M3) were identified. The
current study reports the identification of a rat homologue,
rat gene-3 (R3) relaxin that is highly expressed in a discrete
region of the adult brain. The full R3 relaxin cDNA was generated
using RT-PCR and 3' and 5' RACE protocols. The derived amino
acid sequence of R3 relaxin retains all the characteristic features
of a relaxin peptide and has a high degree of homology with
H3 and M3 relaxin. The distribution of R3 relaxin mRNA in adult
rat brain was determined and highly abundant expression was
only detected in neurons of the ventromedial dorsal tegmental
nucleus (vmDTg) in the pons, whereas all other brain areas were
unlabelled or contained much lower mRNA levels. Relaxin binding
sites and relaxin immunoreactivity were also detected in the
vmDTg. These together with earlier findings provide strong evidence
for a role(s) for multiple relaxin peptides as neurotransmitters
and/or modulators in the rat CNS.
Burazin TC, Bathgate RA, Macris M, Layfield S, Gundlach AL,
Tregear GW. J Neurochem 2002 Sep;82(6):1553-7
Relaxin is an insulin-like peptide consisting of two separate
chains (A and B) joined by two inter- and one intrachain disulfide
bonds. Binding to its receptor requires an Arg-X-X-X-Arg-X-X-Ile
motif in the B-chain. A related member of the insulin superfamily,
INSL3, has a tertiary structure that is predicted to be similar
to relaxin. It also possesses an Arg-X-X-X-Arg motif within
its B-chain, although this is displaced by four amino acids
towards the C-terminus from the corresponding position within
relaxin. We have previously shown that synthetic INSL3 itself
does not display relaxin-like activity although analogue (Analogue
A) with an introduced arginine residue in the B-chain giving
it an Arg cassette in the exact relaxin position does possess
weak activity. In order to identify further the structural features
that impart relaxin function, solid phase peptide synthesis
was used to prepare three additional analogues for bioassay.
Each of these contained point substitutions within the arginine
cassette. Analogue D contained the full human relaxin binding
cassette, Analogue G consisted of the native INSL3 sequence
containing an Arg to Ala substitution, and Analogue E was a
further modification of Analogue A, with the same substitution.
Each analogue was fully chemically characterized by a number
of criteria. Detailed circular dichrosim spectroscopy analyses
showed that the changes caused little alteration of secondary
structure and, hence, overall conformation. However, each analogue
displayed only weak relaxin-like activity. These results indicate
that while the arginine cassette is vital for relaxin-like activity,
there are additional, as yet unidentified structural requirements
for relaxin binding.
Claasz AA, Bond CP, Bathgate RA, Otvos
L, Dawson NF, Summers RJ, Tregear GW, Wade JD. Eur J Biochem
2002 Dec;269(24):6287-93
Several orphan G protein-coupled receptors homologous to gonadotropin
and thyrotropin receptors have recently been identified and
named as LGR4-8. INSL3, also known as Leydig insulin-like peptide
or relaxin-like factor, is a relaxin family member expressed
in testis Leydig cells and ovarian theca and luteal cells. Male
mice mutant for INSL3 exhibit cryptorchidism or defects in testis
descent due to abnormal gubernaculum development whereas overexpression
of INSL3 induces ovary descent in transgenic females. Because
transgenic mice missing the LGR8 gene are also cryptorchid,
INSL3 was tested as the ligand for LGR8. Here, we show that
treatment with INSL3 stimulated cAMP production in cells expressing
recombinant LGR8 but not LGR7. In addition, interactions between
INSL3 and LGR8 were demonstrated following ligand receptor cross-linking.
Northern blot analysis indicated that the LGR8 transcripts are
expressed in gubernaculum whereas treatment of cultured gubernacular
cells with INSL3 stimulated cAMP production and thymidine incorporation.
The present study identified the ligand for an orphan G protein-coupled
receptor based on common phenotypes of ligand and receptor null
mice. Demonstration of INSL3 as the ligand for LGR8 facilitates
understanding of the mechanism of testis descent and allows
studies on the role of INSL3 in gonadal and other physiological
processes.
Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate
RA, Hsueh AJ. J Biol Chem 2002 Aug 30;277(35):31283-6
The relaxin-like factor (RLF), which is the product of the insulin-like
factor 3 (INSL3) gene, is a new circulating peptide hormone
of the relaxin-insulin family. In male mammals, it is a major
secretory product of the testicular Leydig cells, where it appears
to be expressed constitutively but in a differentiation-dependent
manner. In the adult testis, RLF expression is a good marker
for fully differentiated adult-type Leydig cells, but it is
only weakly expressed in prepubertal immature Leydig cells or
in Leydig cells that have become hypertrophic or transformed.
It is also an important product of the fetal Leydig cell population,
where it has been demonstrated using knockout mice to be responsible
for the second phase of testicular descent acting on the gubernaculum.
INSL3 knockout mice are cryptorchid, and in estrogen-induced
cryptorchidism, RLF levels in the testis are significantly reduced.
RLF is also made in female tissues, particularly in the follicular
theca cells of small antral follicles and in the corpus luteum
of the cycle and pregnancy. The ruminant ovary has a very high
level of RLF expression, and analysis of primary cultures of
ovarian theca-lutein cells indicated that, as in the testis,
expression is probably constitutive but differentiation dependent.
Female INSL3 knockout mice have altered estrous cycles, where
RLF may be involved in follicle selection, an idea strongly
supported by observations on bovine secondary follicles. Recently,
a novel 7-transmembrane domain receptor (LGR8 or Great) has
been tentatively identified as the RLF receptor, and its deletion
in mice leads also to cryptorchidism.
Ivell R, Bathgate RA.
Biol Reprod 2002 Sep;67(3):699-705
| Insulin-like 3 is a
new insulin-like circulating hormone. Preliminary result
from our laboratories indicated that the circulating
levels of human Insulin-like 3 in male was 10~12 times
higher than female. |
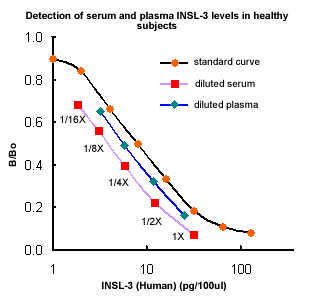
| 
|
| Measurement
of human Insulin-like 3 immunoreactivity
(non-extracted serum and plasma samples)
in healthy subjects: |
| |
Male (Mean ± SD, n=8) |
Female
(Mean ± SD, n=8) |
| Serum |
758.42 ± 83.21
pg/ml |
59.42 ± 25.76
pg/ml |
| Plasma |
594.10 ± 118.7
pg/ml |
47.13 ± 19.84
pg/ml |
April 18, 2003 Copyright Phoenix Pharmaceuticals,
Inc. All Rights Reserved.
|
ChangLu Liu, et al. 4th International Conference on Relaxin
and Related peptides. Sep. 5-10, 2004, Wyoming, USA
|
|
|
Relaxin_E
%INSL%;%Relaxin%
|
|
|


