|
|
|
Serpinin |
Chromogranin A-derived, secreted peptide up-regulates Nexin-1 and granule biogenesis |
Model for intracellular trafficking of granule proteins and autocrine regulation of DCG biogenesis by the CgA-derived peptide serpinin in endocrine cells. Granule proteins (GPs, including granins and prohormones) are synthesized at the RER and then transported to the Golgi complex where they are sorted at the TGN into the regulated secretory pathway. Granins (e.g., CgA) and prohormones form aggregates, which bind to SgIII or carboxypeptidase E (CPE), sorting receptors that are anchored to cholesterol-sphingolipid-rich membrane microdomains at the TGN. These membrane domains bud under the driving force of the granin (CgA and CgB) aggregates to form immature granules. Specific proteolytic enzymes process the prohormones fully or the granins partially to yield biologically active peptides. The granins, the major protein in the granules, condense to form mature DCG. Excess granule proteins are degraded in the Golgi complex. Upon stimulation of the cell, DCG exocytose and release their contents. In cells expressing CgA, a C-terminal peptide, serpinin, is released and binds to a putative G protein-coupled receptor to increase the transcription and biosynthesis of protease nexin-1 (PN-1) via a cAMP-PKA-Sp1 signal transduction pathway. PN-1 inhibits GP degradation to increase GP levels, which in turn leads to more DCG formation to replenish the ones secreted
Figure from: Endocr Rev. 2011 Dec;32(6):755-97. doi: 10.1210/er.2010-0027. Epub 2011 Aug 23. | 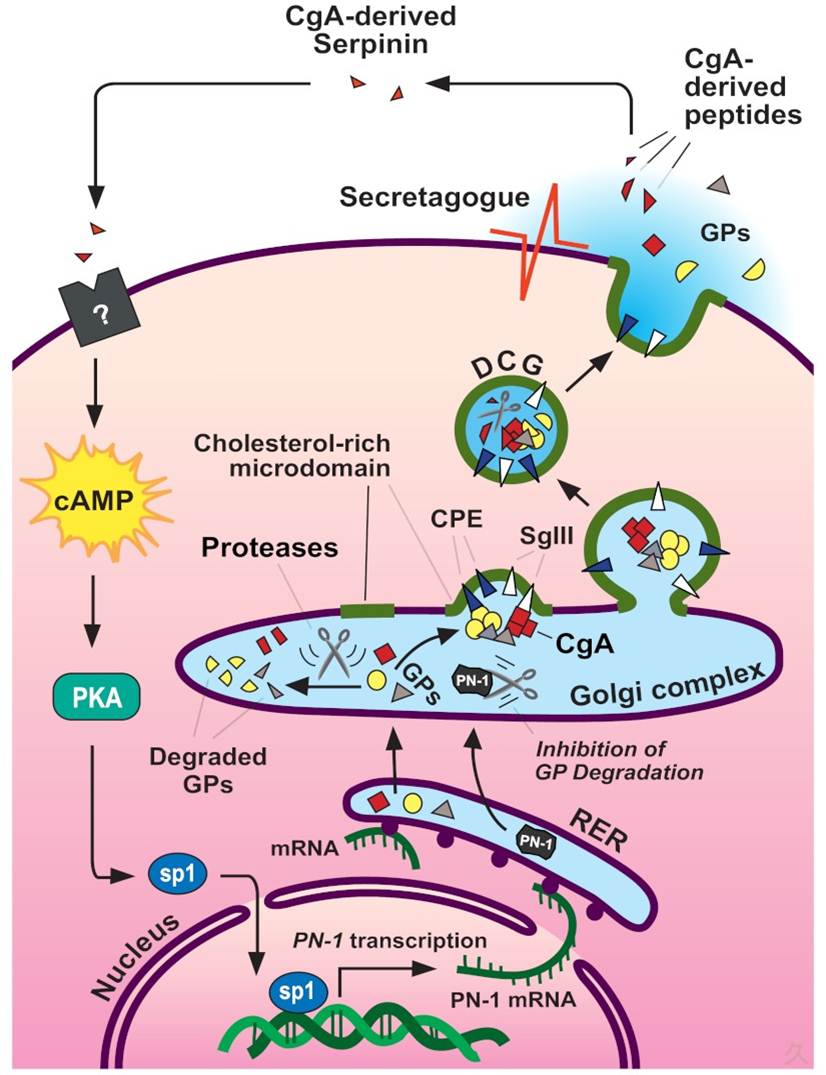 |
Previously we demonstrated that chromogranin A (CgA) promoted secretory granule biogenesis in endocrine cells by stabilizing and preventing granule protein degradation in the Golgi, through up-regulation of expression of the protease inhibitor, protease nexin-1 (PN-1). However, the mechanism by which CgA signals the increase of PN-1 expression is unknown. Here we identified a 2.9-kDa CgA-C-terminus peptide, which we named serpinin, in conditioned media from AtT-20 cells, a corticotroph cell line, which up-regulated PN-1 mRNA expression. Serpinin was secreted from AtT-20 cells upon high potassium stimulation and increased PN-1 mRNA transcription in these cells, in an actinomycin D-inhibitable manner. CgA itself and other CgA-derived peptides, when added to AtT-20 cell media, had no effect on PN-1 expression. Treatment of AtT-20 cells with 10 nm serpinin elevated cAMP levels and PN-1 mRNA expression, and this effect was inhibited by a protein kinase A inhibitor, 6-22 amide. Serpinin and a cAMP analog, 8-bromo-cAMP, promoted the translocation of the transcription factor Sp1 into the nucleus, which is known to drive PN-1 expression. Additionally, an Sp1 inhibitor, mithramycin A inhibited the serpinin-induced PN-1 mRNA up-regulation. Furthermore, a luciferase reporter assay demonstrated serpinin-induced up-regulation of PN-1 promoter activity in an Sp1-dependent manner. When added to CgB-transfected 6T3 cells, a mutant AtT20 cell line, serpinin induced granule biogenesis as evidenced by the presence of CgB puncta accumulation in the processes and tips. Our findings taken together show that serpinin, a novel CgA-derived peptide, is secreted upon stimulation of corticotrophs and plays an important autocrine role in up-regulating PN-1-dependent granule biogenesis via a cAMP-protein kinase A-Sp1 pathway to replenish released granules.
Koshimizu H, Mol Endocrinol. 2011 Mar 24. [Epub ahead of print]
Chromogranin A (CgA), a member of the granin family serves several important cell biological roles in (neuro)endocrine cells which are summarized in this review. CgA is a "prohormone" that is synthesized at the rough endoplasmic reticulum and transported into the cisternae of this organelle via its signal peptide. It is then trafficked to the Golgi complex and then to the trans-Golgi network (TGN) where CgA aggregates at low pH in the presence of calcium. The CgA aggregates provide the physical driving force to induce budding of the TGN membrane resulting in dense core granule (DCG) formation. Within the granule, a small amount of the CgA is processed to bioactive peptides, including a predicted C-terminal peptide, serpinin. Upon stimulation, DCGs undergo exocytosis and CgA and its derived peptides are released. Serpinin, acting extracellularly is able to signal the increase in transcription of a serine protease inhibitor, protease nexin-1 (PN-1) that protects DCG proteins against degradation in the Golgi complex, which then enhances DCG biogenesis to replenish those that were released. Thus CgA and its derived peptide, serpinin, plays a significant role in granule formation and regulation of granule biogenesis, respectively, in (neuro) endocrine cells.
Koshimizu, H. Regul Pept. 2010 Feb 25;160(1-3):153-9. Epub 2009 Dec 16.
Chromogranin A (CgA) is a member of the granin family of molecules found in secretory granules of endocrine and neuro-endocrine cells. Here, we have identified a new 23-mer CgA-derived peptide secreted from pituitary AtT-20 cells, which we named pyroGlu-serpinin (pGlu-serpinin). LC-MS studies of peptides in conditioned medium of AtT-20 cells indicate that pGlu-serpinin is derived from initial processing of mouse CgA at paired basic residues, Arg461-Arg462 and Arg433-Arg434, to yield a previously described 26 amino acid peptide, serpinin. Three amino acids are then cleaved from the N terminus of serpinin, yielding a peptide with an N-terminal glutamine, which is then subsequently pyroglutaminated. Immunocytochemistry showed co-localization of pGlu-serpinin with adrenocorticotropic hormone in secretory granules of AtT-20 cells, and it was released in an activity-dependent manner. Functional studies demonstrated that pGlu-serpinin was able to prevent radical oxygen species (hydrogen peroxide)-induced cell death of AtT-20 cells and cultured rat cerebral cortical neurons at a concentration of 1 and 10 nM, respectively. These data indicate that pGlu-serpinin has anti-apoptotic effects that may be important in neuroprotection of central nervous system neurons and pituitary cells. Furthermore, pGlu-serpinin added to the media of AtT-20 cells up-regulated the transcription of the serine protease inhibitor, protease nexin-1 (PN-1) mRNA. pGlu-serpinin's ability to increase levels of PN-1, a potent inhibitor of plasmin released during inflammatory processes causing cell death, may play a role in protecting cells under adverse pathophysiological conditions.
Koshimizu, H. J. Mol. Neurosci. 2011 May 3. [Epub ahead of print].
|
|
|
pnxnews_000000052;pnxnews_000000487;pnxnews_000000312;
053-31;%053-45%;T-053-47;053-48;T-053-49;053-51;%chromogranin%;%catestatin%;%pancreastatin%
|
|
|


