|
|
|
Urotensin II : A Biomarker in Cardiovascular Diseases |
Y.G. Zhang1, J Yang2, X.H. Wang1, M.G. Fu1, Y. Zhang1, C. S. Tang, J. K. Chang 2
1 Department of Cardiovascular Research, the First Hospital, Beijing Medical University, Beijing 100034, P. R. China;
2 Phoenix Pharmaceuticals, Inc. Belmont, CA, 94002, U. S. A.
Objective : To investigate alteration of functional receptors for human urotensin II (U II) in pressure-overload-induced cardiac hypertrophy of rats.
Methods: In the rat cardiac hypertrophy model produced by constriction of the abdominal aorta, urotensin II binding sites in myocardial sarcolemma were determined with radioligand assay on first day (early group) and 30th day (late group) after operation.
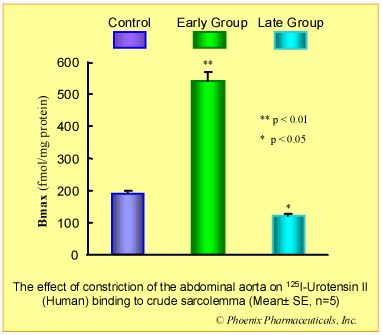
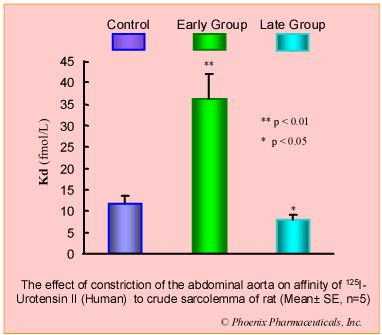
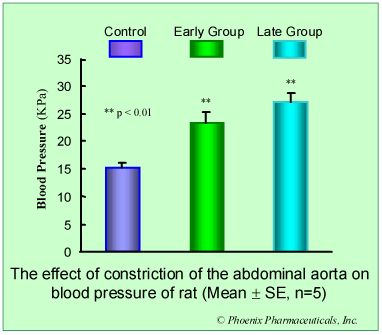
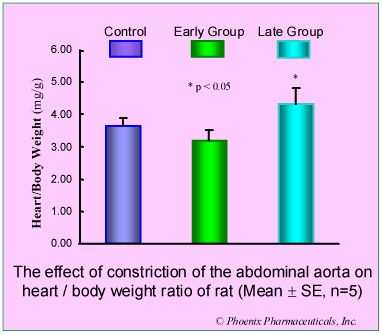
Results: Compared with control group, in early group, the blood pressure increased 50% (p<0.05) and heart/body weight ratio unchanged (p>0.05), U II receptors were up-regulated by 184% (p<0.01) while the affinity to U II was decreased (Kd increased 224%, p<0.01). In late group, the pressure and heart/body weight ratio increased 85% (p<0.01) and 18% (p<0.05) respectively. U II receptors were down-regulated by 35% (p<0.05) while the affinity to U II was increased (Kd decreased 30%, p<0.05).
Conclusion: The biphasic changes of U II receptor in myocardial sarcolemma induced by pressure-overload was observed, increasing in early stage, while decreasing in late stage, and these changes were involved in the development of cardiac hypertrophy.
The cyclic peptide urotensin II (UII) has recently been cloned in mammals and reported to constrict rat pulmonary arteries potently. An enhanced maximal response was shown in rats exposed to chronic hypoxia. The aim of this study was to investigate changes in plasma and myocardial UII levels and its receptor sites in crude sarcolemma of ventricles from chronic hypoxic rats. We observed that rats exposed to chronic hypoxia for 4 weeks developed pulmonary hypertension and right ventricular hypertrophy. Compared with controls, the UII content in hypoxic rats was increased by 97.5% (45.24 +/- 7.1 vs. 22.9 +/- 3.24pg/mg protein, P < 0.01) in the right ventricle and 33.2% (24.89 +/- 0.99 vs. 18.68 +/- 2.04pg/mg protein, P < 0.01) in the left ventricle, respectively. However, there was no significant difference in plasma (27.44 +/- 3.11 vs. 27.82 +/- 5.57pg/ml, P > 0.05) and lung tissue levels (34.03 +/- 4.63 vs. 33.74 +/- 4.06 pg/ mg protein, P > 0.05) between the control and hypoxic groups. The time course of the binding of [125I]UII to crude ventricular sarcolemma was specific and time dependent. Scatchard plot analysis of the data demonstrated that the maximal number of specific binding sites (Bmax) in both the right and left ventricles was upregulated in the hypoxic group. Moreover, Bmax in the right ventricular specimens was upregulated to a greater extent than in the left ventricle (increased by 114% and 25% in the right and left ventricles, respectively, compared with control group, P < 0.01). In contrast, the UII binding affinity in right and left ventricular membranes from hypoxic rats was decreased (the dissociation constant Kd) increased by 20% and 33%, respectively compared with controls, P < 0.01). These results indicate that UII may act as an autocrine and/or paracrine hormone rather than as a circulating hormone, playing important roles in the development of ventricular hypertrophy induced by chronic hypoxia, and that the pathophysiological significance of UII in pulmonary and cardiovascular alteration induced by chronic hypoxia deserves further investigation.
Zhang et al. Heart Vessels. 2002 Jan;16(2):64-8.
The distribution of urotensin-II-immunoreactivity (irU-II) was studied in the rat brainstem and spinal cord with the use of an antiserum against the human urotensin II (U-II) peptide. A population of ventral horn neurons in the spinal cord, hypoglossal nucleus, dorsal motor nucleus of the vagus, facial motor nucleus, nucleus ambiguus, abducens nucleus and trigeminal motor nucleus exhibited irU-II of varying intensities. The number of irU-II motor neurons was higher in the lumbar segments as compared to that of cervical, thoracic and sacral segments. Double-labeling the sections with U-II- and choline acetyltransferase (ChAT)-antisera revealed that nearly all irU-II ventral horn and brainstem neurons were ChAT-positive. The result provides the first immunohistochemical evidence of the presence of irU-II in cholinergic motoneurons of the rat spinal cord and brainstem.
Dun et al.
Neurosci Lett 2001 Jun 1;305(1):9-12.
Urotensin II (UII) is a potent vasoactive cyclic peptide thought to play a role in myocardial hypertrophy and remodelling. We therefore determined UII plasma levels in congestive heart failure (CHF) patients and its relationship with the severity of the disease and well-established markers of left ventricular function. UII was significantly higher in CHF patients (n=57) than in controls (n=48) [geometric mean (pg/ml), 95% PI: 1.32 (0.67-2.59) versus 0.84 (0.31-1.61), p<0.0001], was related to the functional class of the disease and correlated negatively with left ventricular ejection fraction (r=-0.316, P=0.016). Furthermore, UII correlated significantly with Big-ET1 (r=0.32, p=0.03), BNP (r=0.42, p=0.005) but poorly with Nt-proANP (r=0.28, p=0.07). Our results suggest that UII could play a role in worsening the course of congestive heart failure and is associated with established markers of cardiovascular dysfunction.
Gruson et al. Peptides. 2006 Jun;27(6):1527-31.
OBJECTIVE: To investigate the roles of urotensin II (U-II) in chronic obstructive pulmonary disease (COPD).
METHODS: Plasma and induced sputum were obtained from thirty four patients with stable COPD and ten healthy volunteers. The levels of U-II in plasma and induced sputum were measured by RIA kit. Lung function was performed routinely. Induced sputum cells were counted with hemacytometer and differentiated with Wright-Giemsa stain.
RESULTS: The levels of U-II in induced sputum were 82 and 65 folds higher than those of plasma U-II in COPD patients and healthy controls (P<0.01). The levels of U-II in plasma were unrelated to those of induced sputum (r = 0.168, P>0.05). No difference was noted between COPD and healthy controls in the plasma U-II levels [1.46(1.15, 1.73) vs 1.61(1.31, 2.17) microg/L, medians with interquartile ranges, P>0.05]. Sputum U-II levels from COPD patients were 15% higher than those of healthy controls [119.87(105.03, 132.60) vs 104.44 (56.33, 122.24) microg/L, medians with interquartile ranges, P<0.05]. Induced sputum U-II levels of COPD patients had a trend of increase as lung function deteriorated and smoking index increases. Raised sputum total cell and neutrophil counts correlated strongly with the levels of U-II in induced sputum (r = 0.454, r = 0.431, both P<0.01). The levels of U-II in induced sputum correlated negatively with FEV(1)% predicted and p(O(2)) (r = -0.417, r = -0.518, both P<0.05).
CONCLUSION: U-II may act locally, or, via paracrine or autocrine way, play a role in the mechanism of the airway inflammation and airway remodeling in COPD.
Lu et al. Beijing Da Xue Xue Bao. 2006 Apr 18;38(2):155-8.
BACKGROUND: Urotensin II (UTN), a cyclic undecapeptide widely distributed in various organs and tissues, is found in high concentration in atheromatous lesions. Because UTN accumulates in patients with chronic renal failure, the association between plasma UTN and biomarkers of atherosclerosis and endothelial activation needs to be better understood.
METHODS: We tested by a robust statistical approach (Holm method) the association between plasma UTN and biomarkers of atherosclerosis and endothelial activation in a population of 191 patients undergoing chronic hemodialysis.
RESULTS: Plasma UTN was significantly higher in patients with end-stage renal disease (median: 6.5 ng/mL) than in healthy subjects (median: 3.1 ng/mL) (P < .001), and in both patients and control subjects it was independent of age and sex. Interestingly, UTN was inversely related to fibrinogen (r = -0.50, P < .004), intracellular adhesion molecule-1 (r = -0.24, P < .004) and with NO synthesis inhibitor asymmetric dimethyl-arginine (r = -0.40, P < .004). These links were paralleled by direct correlations with albumin (r = 0.21, P < .006) and with transforming growth factor-beta1 (TGFbeta1) (r = 0.36, P < .004). Of note, on multiple regression analysis, these associations remained highly significant also after data adjustment for potential confounders.
CONCLUSIONS: The inverse links between UTN with biomarkers of atherosclerosis and endothelial activation suggest that downregulation of UTN may be a counter-regulatory response aimed at mitigating cardiovascular damage or that UTN itself is a protective factor. Mallamaci F, et al. Am J Hypertens. 2006 May;19(5):505-10 Urotensin II in end-stage renal disease: An inverse correlate of sympathetic function and cardiac natriuretic peptides Urotensin II (UTN) is a peptide highly conserved across species with disparate effects on the vascular system and it is currently unclear whether high plasma UTN levels play a vasculotoxic or a vasculoprotective role. Methods: In this study, we investigated the relationship between plasma UTN and sympathetic activity and cardiac natriuretic hormones in 191 hemodialysis (HD) patients without clinical evidence of heart failure. Results: Plasma UTN was significantly higher in patients with end-stage renal disease (ESRD) (median: 6.5 ng/mL) than in age matched healthy subjects (median: 3.1 ng/mL) (p<0.001). On univariate analysis, UTN was inversely related to heart rate (r=-0.24), dialysis treatment duration (r=-0.27), norepinephrine (r=-0.28), neuropeptide Y (NPY) (r=-0.66), brain natriuretic peptide (BNP) (r=-0.41) and atrial natriuretic peptide (ANP) (r=-0.28) (all p<0.008). Of note, in multiple regression analyses these associations maintained strength similar to that of the corresponding unadjusted correlation coefficients. Conclusions: The inverse links between UTN and neuro-hormonal factors indicate that UTN down-regulation in the presence of high sympathetic activity and high BNP could be a counter-regulatory response aimed at mitigating cardiovascular (CV) damage or that UTN itself acts as a protective factor.
Mallamaci et al. Am J Hypertens. 2006 May;19(5):505-10
The contractile profile of human urotensin-II (hU-II) was examined in primate airway and pulmonary vascular tissues. hU-II contracted tissues from different airway regions with similar potencies (pD(2)s from 8.6 to 9.2). However, there were regional differences in the efficacy of hU-II, with a progressive increase in the maximum contraction from trachea to smaller airway regions (from 9 to 41% of the contraction to 10 muM carbachol). hU-II potently contracted pulmonary artery tissues from different regions with similar potencies and efficacies: pD(2)s=8.7 to 9.3 and maximal contractions=79 to 86% of 60 mM KCl. hU-II potently contracted pulmonary vein preparations taken proximal to the atria, but had no effect in tissues from distal to the atria. This is the first report describing the contractile activity of hU-II in airways and suggests that the potential pathophysiological role of this peptide in lung diseases warrants investigation.
Hay et al. Br J Pharmacol 2000 Sep 1;131(1):10-12
The effects of human urotensin II on coronary flow were studied in the perfused rat heart. Urotensin II transiently decreased coronary flow, then induced sustained vasodilatation. In the presence of a cyclooxygenase inhibitor, diclofenac, coronary vasodilatation was significantly inhibited. A nitric oxide synthase inhibitor, N(G)-nitro-L-arginine (L-NNA), attenuated the urotensin-induced vasodilatation. These data suggest that urotensin II modulates coronary flow through factors such as cyclooxygenase products and nitric oxide to elicit coronary vasodilatation.
Katano et al. Eur J Pharmacol 2000 Aug 18;402(1-2):209-211
The possible role of the endothelium in modulating responses to human urotensin-II (U-II) was investigated using isolated segments of rat thoracic aorta, small mesenteric artery, left anterior descending coronary artery and basilar artery. Human U-II was a potent vasoconstrictor of endothelium-intact isolated rat thoracic aorta (EC50=3.5±1.1 nM, Rmax=103±10% of control contraction induced by 60 mM KCl and 1 µM noradrenaline). However the contractile response was not significantly altered by removal of the endothelium or inhibition of nitric oxide synthesis with L-NAME (100 µM). Human U-II did not cause relaxation of noradrenaline-precontracted, endothelium-intact rat aortae. Human U-II contracted endothelium-intact rat isolated left anterior descending coronary arteries (EC50=1.3±0.8 nM, Rmax=20.1±4.9% of control contraction induced by 10 µM 5-HT). The contractile response was significantly enhanced by removal of the endothelium (Rmax=55.4±16.1%). Moreover, human U-II caused concentration-dependent relaxation of 5-HT-precontracted arteries, which was abolished by L-NAME or removal of the endothelium. No contractile effects of human U-II were found in rat small mesenteric arteries. However the peptide caused potent, concentration- and endothelium-dependent relaxations of methoxamine-precontracted vessels. The relaxant responses were potentiated by L-NAME (300 µM) but abolished in the additional presence of 25 mM KCl (which inhibits the actions of endothelium-derived hyperpolarizing factor). The present study is the first to show that human U-II is a potent endothelium-dependent vasodilator in some rat resistance vessels, and acts through release of EDHF as well as nitric oxide. Our findings have also highlighted clear anatomical differences in the responses of different vascular beds to human U-II which are likely to be important in determining the overall cardiovascular activity of this peptide.
Bottrill et al. Br J Pharmacol. 2000 Aug;130(8):1865-70.
Responses to human urotensin-II (hU-II) were investigated in human and rat pulmonary arteries. Rat pulmonary arteries: hU-II was a potent vasoconstrictor of main pulmonary arteries (2 - 3 mm i.d.) (pEC(50), 8.55+/-0.08, n=21) and was ~4 fold more potent than endothelin-1 [ET-1] (P<0.01), although its E(max) was considerably less (~2.5 fold, P<0.001). The potency of hU-II increased 2.5 fold with endothelium removal (P<0.05) and after raising vascular tone with ET-1 (P<0.01). E(max) was enhanced ~1.5 fold in the presence of N(omega)-nitro-L-arginine methylester (L-NAME, 100 muM, P<0.01) and ~2 fold in vessels from pulmonary hypertensive rats exposed to 2 weeks chronic hypoxia (P<0.05). hU-II did not constrict smaller pulmonary arteries. Human pulmonary arteries (~250 mum i.d.): in the presence of L-NAME, 3 out of 10 vessels contracted to hU-II and this contraction was highly variable. hU-II is, therefore, a potent vasoconstrictor of rat main pulmonary arteries and this response is increased by endothelial factors, vascular tone and onset of pulmonary hypertension. Inhibition of nitric oxide synthase uncovers contractile responses to hU-II in human pulmonary arteries.
MacLean et al. Br J Pharmacol 2000 May 2;130(2):201-204
Urotensin II (UII) is a neuropeptide with potent cardiovascular effects. Its sequence is strongly conserved among different species and has structural similarity to somatostatin. No receptor for UII has been molecularly identified from any species so far. GPR14 was cloned as an orphan G protein-coupled receptor with similarity to members of the somatostatin/opioid receptor family. We have now demonstrated that GPR14 is a high affinity receptor for UII and designate it UII-R1a. HEK293 cells and COS-7 cells transfected with rat GPR14 showed strong, dose-dependent calcium mobilization in response to fish, frog, and human UII. Radioligand binding analysis showed high affinity binding of UII to membrane preparations isolated from HEK293 cells stably expressing rat GPR14. In situ hybridization analysis showed that GPR14 was expressed in motor neurons of the spinal cord, smooth muscle cells of the bladder, and muscle cells of the heart. The identification of the first receptor for UII will allow better understanding of the physiological and pharmacological roles of UII.
Liu et al. Biochem Biophys Res Commun 1999 Dec 9;266(1):174-8
Two molecular species of urotensin II (UII) were isolated from porcine spinal cords and identified as the endogenous ligands of a G-protein-coupled orphan receptor, SENR (sensory epithelium neuropeptide-like receptor), which is identical to GPR14. We established a CHO cell line stably expressing the rat SENR and investigated several tissue extracts to evoke the response mediated by the SENR. Extract from porcine spinal cords showed an activity of arachidonic acid metabolites release from SENR-expressing cells and was purified using HPLC. Two active substances were isolated and their sequences were determined as GPTSECFWKYCV and GPPSECFWKYCV, which were revealed to be porcine UII. Synthetic UII peptides caused arachidonic acid metabolites release activity in the rat SENR-expressing cells with an EC(50) value of 1 nM. Three cDNAs encoding the precursor proteins of porcine UII were cloned from a porcine spinal cord cDNA library; 2 consist of 121 amino acid residues and the other, which seemed to be a splicing variant, consist of 85 residues.
Mori et al. Biochem Biophys Res Commun 1999 Nov;265(1):123-9
Urotensin-II (U-II) is a vasoactive 'somatostatin-like' cyclic peptide which was originally isolated from fish spinal cords, and which has recently been cloned from man. Here we describe the identification of an orphan human G-protein-coupled receptor homologous to rat GPR14 and expressed predominantly in cardiovascular tissue, which functions as a U-II receptor. Goby and human U-II bind to recombinant human GPR14 with high affinity, and the binding is functionally coupled to calcium mobilization. Human U-II is found within both vascular and cardiac tissue (including coronary atheroma) and effectively constricts isolated arteries from non-human primates. The potency of vasoconstriction of U-II is an order of magnitude greater than that of endothelin-1, making human U-II the most potent mammalian vasoconstrictor identified so far. In vivo, human U-II markedly increases total peripheral resistance in anaesthetized non-human primates, a response associated with profound cardiac contractile dysfunction. Furthermore, as U-II immunoreactivity is also found within central nervous system and endocrine tissues, it may have additional activities.
Ames et al. Nature. 1999 Sep 16;401(6750):282-6.
Urotensin II (UII) is a cyclic neuropeptide initially isolated from the caudal neurosecretory system of teleost fish. The recent cloning of the UII precursor in frog and human has demonstrated that the peptide is not restricted to the fish urophysis but that it is also expressed in the central nervous system of tetrapods. Here, we describe the characterization of the cDNAs encoding prepro-UII in mouse and rat. A comparison of the primary structures of mouse and rat UII with those of other vertebrate UII reveals that the sequence of the cyclic region of the molecule (CFWKYC) has been fully conserved. In contrast, the N-terminal flanking domain of prepro-UII has markedly diverged with only 48% sequence identity between the mouse or rat and the human precursors. In situ hybridization histochemistry showed that the prepro-UII gene is predominantly expressed in motoneurons of the brainstem and spinal cord, suggesting that UII may play a role in the control of neuromuscular functions.
Coulouarn et al. FEBS Lett 1999 Aug 20;457(1):28-32
Urotensin II (UII) is a cyclic peptide initially isolated from the caudal neurosecretory system of teleost fish. Subsequently, UII has been characterized from a frog brain extract, indicating that a gene encoding a UII precursor is also present in the genome of a tetrapod. Here, we report the characterization of the cDNAs encoding frog and human UII precursors and the localization of the corresponding mRNAs. In both frog and human, the UII sequence is located at the C-terminal position of the precursor. Human UII is composed of only 11 amino acid residues, while fish and frog UII possess 12 and 13 amino acid residues, respectively. The cyclic region of UII, which is responsible for the biological activity of the peptide, has been fully conserved from fish to human. Northern blot and dot blot analysis revealed that UII precursor mRNAs are found predominantly in the frog and human spinal cord. In situ hybridization studies showed that the UII precursor gene is actively expressed in motoneurons. The present study demonstrates that UII, which has long been regarded as a peptide exclusively produced by the urophysis of teleost fish, is actually present in the brain of amphibians and mammals. The fact that evolutionary pressure has acted to conserve fully the biologically active sequence of UII suggests that the peptide may exert important physiological functions in humans.
Coulouarn et al. Proc Natl Acad Sci U S A 1998 Dec 22;95(26):15803-8
The aim of this study was to observe the effects of adrenomedullin (ADM) on endothelin (ET) production induced by urotensin II (U II) in rat vascular smooth muscle cells (VSMCs). Cultured VSMCs which were incubated with UII (10-8 mol/L) and with various concentrations of ADM were used to measure the VSMCs 3H-TdR incorpora- tion, the activity of extracellular signal-regulated kinase (ERK), the amount of ET mRNA and ET production in VSMCs. In this work we found that incubation with UⅡ(10-8 mol/L) increased obviously the amount of ET mRNA in VSMCs and ET production in medium, however, coincubation with ADM (10-10—10-8 mol/L) and UII(10-8 mol/L) reduced the amount of ET mRNA by 15%, 24% and 45% (P< 0.01) respectively, compared with UII alone. The content of ET in medium was 14.13, 11.38 and 11.00 pg/mL. ADM alone (10-8 mol/L) had no effect on ET production in VSMCs. UII (10-8 mol/L) promoted the 3H-TdR incorpo- ration and activity of ERK in VSMCs. ADM inhibited VSMCs 3H-TdR incorporation and activation of ERK in a concentration-dependent manner. Compared with UII group, after coincubation with ADM (10-10—10-8 mol/L) and UII (10-8 mol/L) the VSMCs 3H-TdR incorporation was decreased by 7% (P > 0.05), 32% (P < 0.05) and 41% (P < 0.01), respectively, and the activity of ERK was decreased by 24% (P > 0.05), 32% (P < 0.05) and 36% (P < 0.05), re- spectively, in a concentration-dependent manner. The results show that in cultured VSMCs ADM inhibits ET mRNA expression, ET production and proliferation stimulated by UII, and that inhibitory effect of ADM on UⅡ bioaction could be mediated through inhibiting MAPK pathway.
Yongfen et al. Chinese Science Bulletin ISSN:1001-6538 2002 Vol.47 No.17 1457-1461
|
|
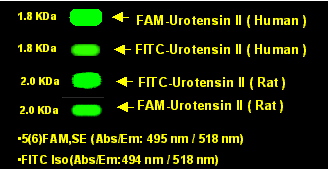
Electrophoresis |
15% Tricine-PAGE gel |
Blocking |
No primary antibody |
Negative Control |
1% BSA in TBST or 5% dry milk in TBST |
Primary Antibody |
Rabbit Anti-Urotensin, Prepro (71-84)(Human) Antiserum (Catalog No.:H-071-22) |
Optimal Dilution |
1:500 (4 hour at RT, or overnight at 4 0 C) |
Secondary Antibody |
Donkey anti-Rabbit IgG whole (H+L), HRP conjugate(1:5000, 30 min) |
Detection system |
HRP |
Substrate |
SuperSignal Chemiluminescent Substrate |
|
|
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
Pre-immuno serum |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
Rabbit Anti-urotensin II, Prepro (71-84) (Human) Serum (Catalog No.:H-071-22) |
Optimal Dilution |
1:500 (over night at 4°C) |
Secondary Antibody |
Goat anti-Rabbit IgG, Biotinylated (1:400, 30 min) |
Amplification |
ABC (Vector) (1:400, 30 min) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
|
|
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
Pre-immuno serum |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
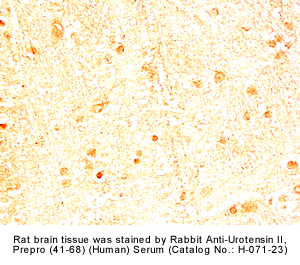
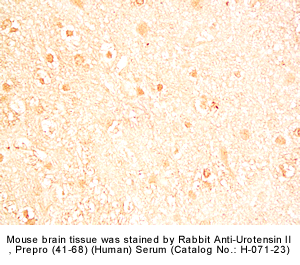
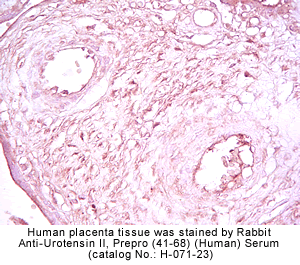
Rabbit Anti-urotensin II, Prepro (41-68) (Human) Serum (Catalog No.:H-071-23) |
Optimal Dilution |
1:500 (over night at 4°C) |
Secondary Antibody |
Goat anti-Rabbit IgG, Biotinylated (1:400, 30 min) |
Amplification |
ABC (Vector) (1:400, 30 min) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
|
|
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
Pre-immuno serum |
Pretreatment |
Intact |
Blocking |
2% Normal Goat Serum |
Primary Antibody |
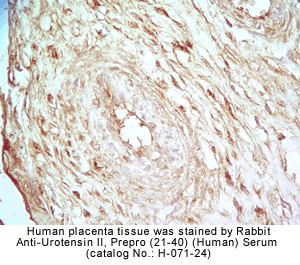
Rabbit Anti-urotensin II, Prepro (41-68) (Human) Serum (Catalog No.:H-071-23)
Rabbit Anti-Urotensin II, Prepro (21-40) (Human) Serum (Catalog No.:H-071-24)
|
Optimal Dilution |
1:500 (over night at 4°C) |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
ABC (Vector) (1:400, 30 min) |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
See More Research Abstracts, Antibody Stainings, Immunoassay Kits Curves and Sequences by Clicking the Tabs on the Top. |
|
|
%Urotensin II%
|
|
|


