|
|
| f-MLF analogs |
strongly stimulate Formyl Peptide Receptors |
Formyl peptide receptors (FPRs) are G-protein-coupled receptors (GPCRs) that function as chemoattractant receptors in innate immune responses. Here we perform systematic structure-function analyses of FPRs from six mammalian species using structurally diverse FPR peptide agonists and identify a common set of conserved agonist properties with typical features of pathogen-associated molecular patterns. Guided by these results, we discover bacterial signal peptides, normally used to translocate proteins across cytoplasmic membranes, as a vast family of natural FPR agonists. N-terminally formylated signal peptides fragments with variable sequence and length activate human and mouse FPR1 and FPR2 at low nanomolar concentrations, thus establishing FPR1 and FPR2 as sensitive and broad signal peptide receptors. The vomeronasal receptor mFpr-rs1 and its sequence orthologue hFPR3 also react to signal peptides but are much more narrowly tuned in signal peptide recognition. Furthermore, all signal peptides examined here function as potent activators of the innate immune system. They elicit robust, FPR-dependent calcium mobilization in human and mouse leukocytes and trigger a range of classical innate defense mechanisms such as the production of reactive oxygen species, metalloprotease release, and chemotaxis. Thus, bacterial signal peptides constitute a novel class of immune activators that are likely to contribute to mammalian immune defense against bacteria. This evolutionary conserved detection mechanism combines structural promiscuity with high specificity, and enables discrimination between bacterial and eukaryotic signal sequences. With at least 175,542 predicted sequences, bacterial signal peptides represent the largest and structurally most heterogeneous class of GPCR agonists currently known for the innate immune system.
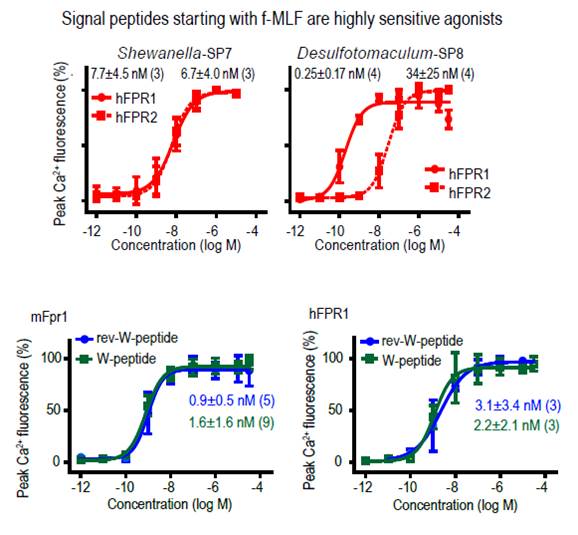
Bufe B, Schumann T, Kappl R et al., J Biol Chem. 2015 Jan 20. pii: jbc.M114.626747. [Epub ahead of print]
Bacterial/mitochondrial fMLF analogs bind FPR1, driving accumulation/activation of PMN at sites of infection/injury, while promoting wound healing in epithelia. We quantified levels of UFPR1 and TFPR1 in isolated PMN by use of phosphosensitive NFPRb and phosphorylation-independent NFPRa antibodies. UFPR1 and total TFPR were assessed inflamed mucosa, observed in human IBD. In isolated PMN after fMLF stimulation, UFPR1 declined 70% ((fMLF)EC50 = 11 ± 1 nM; t1/2 = 15 s) and was stable for up to 4 h, whereas TFPR1 changed only slightly. Antagonists (tBoc-FLFLF, CsH) and metabolic inhibitor NaF prevented the fMLF-dependent UFPR1 decrease. Annexin A1 fragment Ac2-26 also induced decreases in UFPR1 ((Ac2-26)EC50 ∼ 3 µM). Proinflammatory agents (TNF-α, LPS), phosphatase inhibitor (okadaic acid), and G-protein activator (MST) modestly increased (fMLF)EC50, 2- to 4-fold, whereas PTX, Ca(2+) chelators (EGTA/BAPTA), H2O2, GM-CSF, ENA-78, IL-1RA, and LXA4 had no effect. Aggregation-inducing PAF, however, strongly inhibited fMLF-stimulated UFPR1 decreases. fMLF-driven PMN also demonstrated decreased UFPR1 after traversing monolayers of cultured intestinal epithelial cells, as did PMN in intestinal mucosal samples, demonstrating active inflammation from UC patients. Total TFPR remained high in PMN within inflamed crypts, migrating through crypt epithelium, and in the lamina propria-adjoining crypts, but UFPR1 was only observed at some peripheral sites on crypt aggregates. Loss of UFPR1 in PMN results from C-terminal S/T phosphorylation. Our results suggest G protein-insensitive, fMLF-dependent FPR1 phosphorylation in isolated suspension PMN, which may manifest in fMLF-driven transmigration and potentially, in actively inflamed tissues, except at minor discrete surface locations of PMN-containing crypt aggregates.
Leoni G, Gripentrog J, Lord C et al., J Leukoc Biol. 2015 Jan;97(1):87-101. doi: 10.1189/jlb.4A0314-153R. Epub 2014 Nov 13.
The N-formyl peptide receptors (FPRs) are a family of G-protein coupled receptors that respond to proinflammatory N-formylated bacterial peptides (e.g., formyl-Met-Leu-Phe, fMLF) and, thus, contribute to the host response to bacterial infection. Paradoxically, a growing body of evidence suggests that some members of this receptor family may also be targets for certain anti-inflammatory molecules, including annexin A1 (ANXA1), which is an important mediator of glucocorticoid (GC) action. To explore further the potential role of FPRs in mediating ANXA1 actions, we have focused on the pituitary gland, where ANXA1 has a well-defined role as a cell-cell mediator of the inhibitory effects of GCs on the secretion of corticotrophin (ACTH), and used molecular, genetic, and pharmacological approaches to address the question in well-established rodent models. Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis identified mRNAs for four FPR family members in the mouse anterior pituitary gland, Fpr-rs1, Fpr-rs2, Fpr-rs6, and Fpr-rs7. Functional studies confirmed that, like dexamethasone, ANXA1 and two ANXA1-derived peptides (ANXA1(1-188) and ANXA1(Ac2-26)) inhibit the evoked release of ACTH from rodent anterior pituitary tissue in vitro. Fpr1 gene deletion failed to modify the pituitary responses to dexamethasone or ANXA1(Ac2-26). However, lipoxin A4 (LXA4, 0.02-2 microM, a lipid mediator with high affinity for Fpr-rs1) mimicked the inhibitory effects of ANXA1 on ACTH release as also did fMLF in high (1-100 microM) but not lower (10-100 nM) concentrations. Additionally, a nonselective FPR antagonist (Boc1, 100 microM) overcame the effects of dexamethasone, ANXA1(1-188), ANXA1(Ac2-26), fMLF, and LXA4 on ACTH release, although at a lower concentration (50 microM), it was without effect. Together, the results suggest that the actions of ANXA1 in the pituitary gland are independent of Fpr1 but may involve other FPR family members, in particular, Fpr-rs1 or a closely related receptor. They thus provide the first evidence for a role of the FPR family in the regulation of neuroendocrine function.
John CD, Sahni V, Mehet D, et al., FASEB J. 2007 Apr;21(4):1037-46. Epub 2007 Jan 11.
Most immunomodulatory materials (e.g., vaccine adjuvants such as alum) modulate adaptive immunity, and yet little effort has focused on developing materials to regulate innate immunity, which get mentioned only when inflammation affects the biocompatibility of biomaterials. Traditionally considered as short-lived effector cells from innate immunity primarily for the clearance of invading microorganisms without specificity, neutrophils exhibit a key role in launching and shaping the immune response. Here we show that the incorporation of unnatural amino acids into a well-known chemoattractant-N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLF)-offers a facile approach to create a de novo, multifunctional chemoattractant that self-assembles to form supramolecular nanofibrils and hydrogels. This de novo chemoattractant not only exhibits preserved cross-species chemoattractant activity to human and murine neutrophils, but also effectively resists proteolysis. Thus, its hydrogel, in vivo, releases the chemoattractant and attracts neutrophils to the desired location in a sustainable manner. As a novel and general approach to generate a new class of biomaterials for modulating innate immunity, this work offers a prolonged acute inflammation model for developing various new applications.
Zhao F, Li J, Zhou N, Sakai J et al., Bioconjug Chem. 2014 Dec 17;25(12):2116-22. doi: 10.1021/bc5004923. Epub 2014 Nov 25.
Formyl peptide receptors (FPRs) are a small group of seven-transmembrane domain, G protein-coupled receptors that are expressed mainly by mammalian phagocytic leukocytes and are known to be important in host defense and inflammation. The three human FPRs (FPR1, FPR2/ALX, and FPR3) share significant sequence homology and are encoded by clustered genes. Collectively, these receptors bind an extraordinarily numerous and structurally diverse group of agonistic ligands, including N-formyl and nonformyl peptides of different composition, that chemoattract and activate phagocytes. N-formyl peptides, which are encoded in nature only by bacterial and mitochondrial genes and result from obligatory initiation of bacterial and mitochondrial protein synthesis with N-formylmethionine, is the only ligand class common to all three human receptors. Surprisingly, the endogenous anti-inflammatory peptide annexin 1 and its N-terminal fragments also bind human FPR1 and FPR2/ALX, and the anti-inflammatory eicosanoid lipoxin A4 is an agonist at FPR2/ALX. In comparison, fewer agonists have been identified for FPR3, the third member in this receptor family. Structural and functional studies of the FPRs have produced important information for understanding the general pharmacological principles governing all leukocyte chemoattractant receptors. This article aims to provide an overview of the discovery and pharmacological characterization of FPRs, to introduce an International Union of Basic and Clinical Pharmacology (IUPHAR)-recommended nomenclature, and to discuss unmet challenges, including the mechanisms used by these receptors to bind diverse ligands and mediate different biological functions.
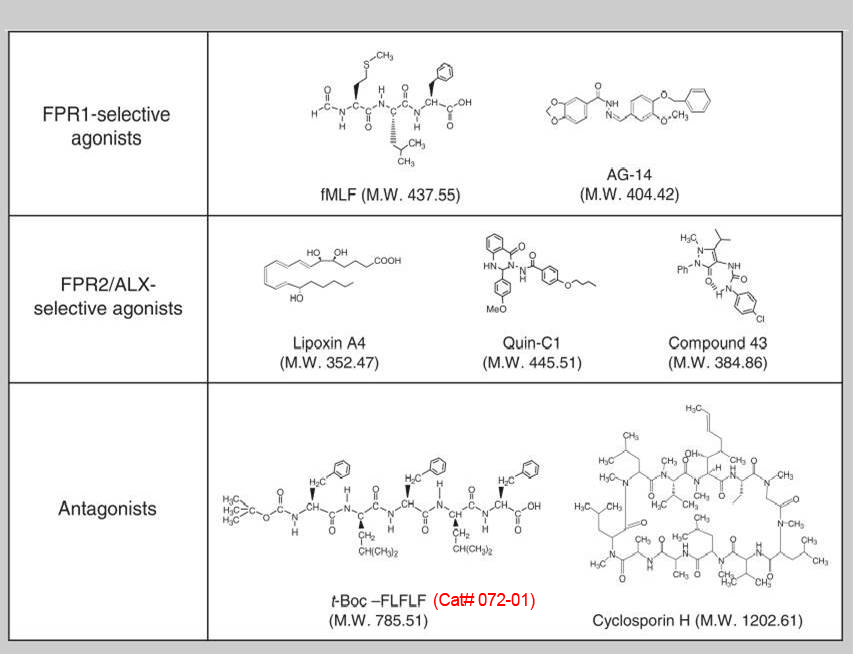
Ye RD, Boulay F, Wang JM, et al., Pharmacol Rev. 2009 Jun;61(2):119-61. doi: 10.1124/pr.109.001578. Epub 2009 Jun 4.
Annexin A1 (ANXA1), a mediator of the anti-inflammatory action of glucocorticoids, is important in cancer development and progression, whereas NF-κB regulates multiple cellular phenomena, some of them associated with inflammation and cancer. We showed that glucocorticoids and chemopreventive modified nonsteroidal anti-inflammatory drugs, such as nitric oxide–donating aspirin (NO-ASA) and phospho-aspirin, induced ANXA1 in cultured human colon and pancreatic cancer cells. ANXA1 associated with NF-κB and suppressed its transcriptional activity by preventing NF-κB binding to DNA. The induction of ANXA1 by glucocorticoids was proportional to their anti-inflammatory potency, as was the suppression of NF-κB activity, which was accompanied by enhanced apoptosis and inhibition of cell growth mediated by changes in NF-κB–dependent cell signaling. The proposed novel mechanism was operational in the intestinal mucosa of mice treated with dexamethasone or NO-ASA. ANXA1-based oligopeptides displayed the same effects as ANXA1 on NF-κB. One such tripeptide (Gln-Ala-Trp) (Cat. # 072-79) administered to nude mice inhibited the growth of SW480 human colon cancer xenografts by 58% compared with control (P < 0.01). Our findings reveal that ANXA1 is an inducible endogenous inhibitor of NF-κB in human cancer cells and mice, provide a novel molecular mechanism for the action of anti-inflammatory agents, and suggest the possibility of mechanism-driven drug development.
Zhiquan Zhang, Liqun Huang, Wenping Zhao, et al., Cancer Res. 2010 Mar 15;70(6):2379-88.
The anti-inflammatory peptide annexin-1 binds to formyl peptide receptors (FPR) but little is known about its mechanism of action in the vasculature. Here we investigate the effect of annexin peptide Ac2-26 on NADPH oxidase activity induced by tumour necrosis factor alpha (TNFα) in human endothelial cells. Superoxide release and intracellular reactive oxygen species (ROS) production from NADPH oxidase was measured with lucigenin-enhanced chemiluminescence and 2',7'-dichlorodihydrofluorescein diacetate, respectively. Expression of NADPH oxidase subunits and intracellular cell adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) were determined by real-time PCR and Western blot analysis. Promoter activity of nuclear factor kappa B (NFκB) was measured by luciferase activity assay. TNFα stimulated NADPH-dependent superoxide release, total ROS formation and expression of ICAM-1and VCAM-1. Pre-treatment with N-terminal peptide of annexin-1 (Ac2-26, 0.5-1.5 µM) reduced all these effects, and the inhibition was blocked by the FPRL-1 antagonist WRW4. Furthermore, TNFα-induced NFκB promoter activity was attenuated by both Ac2-26 and NADPH oxidase inhibitor diphenyliodonium (DPI). Surprisingly, Nox4 gene expression was reduced by TNFα whilst expression of Nox2, p22phox and p67phox remained unchanged. Inhibition of NADPH oxidase activity by either dominant negative Rac1 (N17Rac1) or DPI significantly attenuated TNFα-induced ICAM-1and VCAM-1 expression. Ac2-26 failed to suppress further TNFα-induced expression of ICAM-1and VCAM-1 in N17Rac1-transfected cells. Thus, Ac2-26 peptide inhibits TNFα-activated, Rac1-dependent NADPH oxidase derived ROS formation, attenuates NFκB pathways and ICAM-1 and VCAM-1 expression in endothelial cells. This suggests that Ac2-26 peptide blocks NADPH oxidase activity and has anti-inflammatory properties in the vasculature which contributes to modulate in reperfusion injury inflammation and vascular disease.
Peshavariya HM1, Taylor CJ, Goh C et al., PLoS One. 2013 Apr 24;8(4):e60790. doi: 10.1371/journal.pone.0060790. Print 2013.
|
|
|
AC2-26;W6M;WRW4;Antimicrobial peptides;Ac2-26_MKYMVm
072-77;072-78;072-79;072-80;072-81;%072-0%;072-11;072-12;072-16;072-17;072-20
|
|
|


