|
|
| Myostatin (GDF-8) and GDF-11 Prodomain |
Potential to treat muscle atrophy |
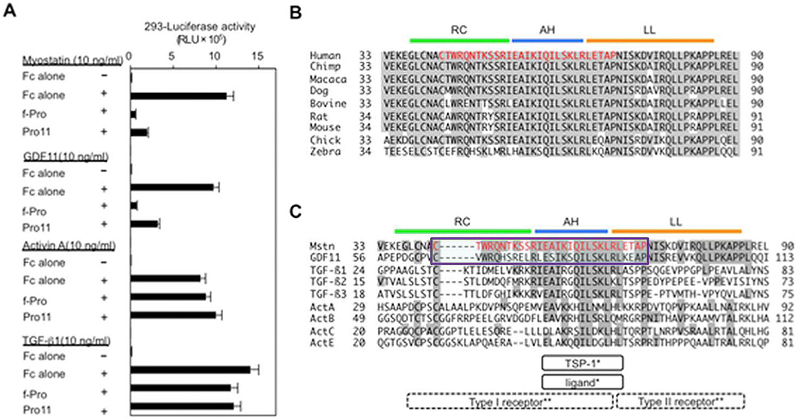
(A) The full-length myostatin prodomain (f-Pro) and its inhibitory core (Pro11) inhibited the transcriptional activities of myostatin and GDF11, but not of TGF-β1 or activin A, in HEK293 cells. (B, C) Sequence alignment of the prodomains of myostatin in nine species (B) and nine TGF-β family members (C). Red indicates the identified inhibitory core of the myostatin prodomain, consisting of 29 amino acids. The AH structure (blue) of the TGF-β1 prodomain has been shown to bind to both its ligand and TSP-1 . Crystallographic analyses of TGF-β1 and its receptors have predicted that the random coiled structure (RC, green) and the AH are located closely to its type I receptor, whereas the latency lasso structure (LL, brown) is located close to its type II receptor .
Figure from: Ohsawa Y, Takayama K, Nishimatsu Si, Okada T, Fujino M, et al. (2015) The Inhibitory Core of the Myostatin Prodomain: Its Interaction with Both Type I and II Membrane Receptors, and Potential to Treat Muscle Atrophy. PLoS ONE 10(7): e0133713. doi:10.1371/journal.pone.0133713
Myostatin, a muscle-specific transforming growth factor-β (TGF-β), negatively regulates skeletal muscle mass. The N-terminal prodomain ofmyostatin noncovalently binds to and suppresses the C-terminal mature domain (ligand) as an inactive circulating complex. However, which region of the myostatin prodomain is required to inhibit the biological activity of myostatin has remained unknown. We identified a 29-amino acid region that inhibited myostatin-induced transcriptional activity by 79% compared with the full-length prodomain. This inhibitory core resides near the N-terminus of the prodomain and includes an α-helix that is evolutionarily conserved among other TGF-β family members, but suppresses activation ofmyostatin and growth and differentiation factor 11 (GDF11) that share identical membrane receptors. Interestingly, the inhibitory core co-localized and co-immunoprecipitated with not only the ligand, but also its type I and type II membrane receptors. Deletion of the inhibitory core in the full-length prodomain removed all capacity for suppression of myostatin. A synthetic peptide corresponding to the inhibitory core (p29) ameliorates impaired myoblast differentiation induced by myostatin and GDF11, but not activin or TGF-β1. Moreover, intramuscular injection of p29 alleviated muscle atrophy and decreased the absolute force in caveolin 3-deficient limb-girdle muscular dystrophy 1C model mice. The injection suppressed activation of myostatin signaling and restored the decreased numbers of muscle precursor cells caused by caveolin 3 deficiency. Our findings indicate a novel concept for this newly identified inhibitory core of the prodomain of myostatin: that it not only suppresses the ligand, but also prevents two distinct membrane receptors from binding to the ligand. This study provides a strong rationale for the use of p29 in the amelioration of skeletal muscle atrophy in various clinical settings.
Ohsawa Y, Takayama K, Nishimatsu S et al., PLoS One. 2015 Jul 30;10(7):e0133713. doi: 10.1371/journal.pone.0133713. eCollection 2015.
The fatal X-linked Duchenne muscular dystrophy (DMD), characterized by progressive muscle wasting and muscle weakness, is caused by mutations within the DMD gene. The use of antisense oligonucleotides (AOs) modulating pre-mRNA splicing to restore the disrupted dystrophin reading frame, subsequently generating a shortened but functional protein has emerged as a potential strategy in DMD treatment. AO therapy has recently been applied to induce out-of-frame exon skipping of myostatin pre-mRNA, knocking-down expression of myostatin protein, and such an approach is suggested to enhance muscle hypertrophy/hyperplasia and to reduce muscle necrosis. Within this study, we investigated dual exon skipping of dystrophin and myostatin pre-mRNAs using phosphorodiamidate morpholino oligomers conjugated with an arginine-rich peptide (B-PMOs). Intraperitoneal administration of B-PMOs was performed in neonatal mdx males on the day of birth, and at weeks 3 and 6. At week 9, we observed in treated mice (as compared to age-matched, saline-injected controls) normalization of muscle mass, a recovery in dystrophin expression, and a decrease in muscle necrosis, particularly in the diaphragm. Our data provide a proof of concept for antisense therapy combining dystrophin restoration and myostatin inhibition for the treatment of DMD.
Lu-Nguyen NB, Jarmin SA, Saleh AF et al., Mol Ther. 2015 Aug;23(8):1341-8. doi: 10.1038/mt.2015.88. Epub 2015 May 11.
Myostatin, an endogenous negative regulator of skeletal muscle mass, is a therapeutic target for muscle atrophic disorders. Here, we identified minimum peptides 2 and 7 to effectively inhibit myostatin activity, which consists of 24 and 23 amino acids, respectively, derived from mouse myostatin prodomain. These peptides, which had the propensity to form α-helix structure, interacted to myostatin with KD values of 30-36 nM. Moreover, peptide 2 significantly increased muscle mass in Duchenne muscular dystrophy-model mice.
Takayama K, Noguchi Y, Aoki S et al., J Med Chem. 2015 Jan 8. [Epub ahead of print]
Emerging evidence indicates that there are factors within the blood of young animals that have the ability to restore youthful characteristics to a number of organ systems in older animals. Growth/differentiation factor 11 (GDF11) is the first of such factors to be identified, and two new studies demonstrate that this "factor of youth" rejuvenates stem cells found in the skeletal muscle and brain of aged mice.
Andersen RE, Lim DA, Cell Res. 2014 Dec;24(12):1381-2. doi: 10.1038/cr.2014.107. Epub 2014 Aug 12.
In the adult central nervous system, the vasculature of the neurogenic niche regulates neural stem cell behavior by providing circulating and secreted factors. Age-related decline of neurogenesis and cognitive function is associated with reduced blood flow and decreased numbers of neural stem cells. Therefore, restoring the functionality of the niche should counteract some of the negative effects of aging. We show that factors found in young blood induce vascular remodeling, culminating in increased neurogenesis and improved olfactory discrimination in aging mice. Further, we show that GDF11 alone can improve the cerebral vasculature and enhance neurogenesis. The identification of factors that slow the age-dependent deterioration of the neurogenic niche in mice may constitute the basis for new methods of treating age-related neurodegenerative and neurovascular diseases.
Katsimpardi L, Litterman NK, Schein PA et al., Science. 2014 May 9;344(6184):630-4. doi: 10.1126/science.1251141. Epub 2014 May 5.
Parabiosis experiments indicate that impaired regeneration in aged mice is reversible by exposure to a young circulation, suggesting that young blood contains humoral "rejuvenating" factors that can restore regenerative function. Here, we demonstrate that the circulating protein growth differentiation factor 11 (GDF11) is a rejuvenating factor for skeletal muscle. Supplementation of systemic GDF11 levels, which normally decline with age, by heterochronic parabiosis or systemic delivery of recombinant protein, reversed functional impairments and restored genomic integrity in aged muscle stem cells (satellite cells). Increased GDF11 levels in aged mice also improved muscle structural and functional features and increased strength and endurance exercise capacity. These data indicate that GDF11 systemically regulates muscle aging and may be therapeutically useful for reversing age-related skeletal muscle and stem cell dysfunction.
Sinha M, Jang YC, Oh J et al, Science. 2014 May 9;344(6184):649-52. doi: 10.1126/science.1251152. Epub 2014 May 5.
All transforming growth factor-β (TGF-β) ligands are synthesised as precursor molecules consisting of a signal peptide, an N-terminal prodomain and a C-terminal mature domain. During synthesis, prodomains interact non-covalently with mature domains, maintaining the molecules in a conformation competent for dimerisation. Dimeric precursors are cleaved by proprotein convertases, and TGF-β ligands are secreted from the cell non-covalently associated with their prodomains. Extracellularly, prodomains localise TGF-β ligands within the vicinity of their target cells via interactions with extracellular matrix proteins, including fibrillin and perlecan. For some family members (TGF-β1, TGF-β2, TGF-β3, myostatin, GDF-11 and BMP-10), prodomains bind with high enough affinity to suppress biological activity. The subsequent mechanism of activation of these latent TGF-β ligands varies according to cell type and context, but all activating mechanisms directly target prodomains. Thus, prodomains control many aspects of TGF-β superfamily biology, and alterations in prodomain function are often associated with disease.
Harrison CA, Al-Musawi SL, Walton KL. Growth Factors. 2011 Oct;29(5):174-86. doi: 10.3109/08977194.2011.608666. Epub 2011 Aug 24.
The specific functions of the prodomains of TGFβ superfamily members are largely unknown. Interactions are known between prodomains of TGFβ-1-3 and latent TGFβ-binding proteins and between prodomains of BMP-2, -4, -7, and -10 and GDF-5 and fibrillins, raising the possibility that latent TGFβ-binding proteins and fibrillins may mediate interactions with all other prodomains of this superfamily. This possibility is tested in this study. Results show that the prodomain of BMP-5 interacts with the N-terminal regions of fibrillin-1 and -2 in a site similar to the binding sites for other bone morphogenetic proteins. However, in contrast, the prodomain of GDF-8 (myostatin) interacts with the glycosaminoglycan side chains of perlecan. The binding site for the GDF-8 prodomain is likely the heparan sulfate chain present on perlecan domain V. These results support and extend the emerging concept that TGFβ superfamily prodomains target their growth factor dimers to extracellular matrix macromolecules. In addition, biochemical studies of prodomain·growth factor complexes were performed to identify inactive complexes. For some members of the superfamily, the prodomain is noncovalently associated with its growth factor dimer in an inactive complex; for others, the prodomain·growth factor complex is active, even though the prodomain is noncovalently associated with its growth factor dimer. Results show that the BMP-10 prodomain, in contrast to BMP-4, -5, and -7 prodomains, can inhibit the bioactivity of the BMP-10 growth factor and suggest that the BMP-10 complex is like TGFβ and GDF-8complexes, which can be activated by cleavage of the associated prodomain.
Sengle G, Ono RN, Sasaki T, Sakai LY. J Biol Chem. 2011 Feb 18;286(7):5087-99. doi: 10.1074/jbc.M110.188615. Epub 2010 Dec 6.
Humoral and tumoral factors collectively promote cancer-induced skeletal muscle wasting by increasing protein degradation. Although several humoral proteins, namely TNFα (tumour necrosis factor α) and IL (interleukin)-6, have been shown to induce skeletal muscle wasting, there is a lack of information regarding the tumoral factors that contribute to the atrophy of muscle during cancer cachexia. Therefore, in the present study, we have characterized the secretome of C26 colon cancer cells to identify the tumoral factors involved in cancer-induced skeletal muscle wasting. In the present study, we show that myostatin, a procachectic TGFβ (transforming growth factor β) superfamily member, is abundantly secreted by C26 cells. Consistent with myostatin signalling during cachexia, treating differentiated C2C12 myotubes with C26 CM (conditioned medium) resulted in myotubular atrophy due to the up-regulation of muscle-specific E3 ligases, atrogin-1 and MuRF1 (muscle RING-finger protein 1), and enhanced activity of the ubiquitin-proteasome pathway. Furthermore, the C26 CM also activated ActRIIB (activin receptor type II B)/Smad and NF-κB (nuclear factor κB) signalling, and reduced the activity of the IGF-I (insulin-like growth factor 1)/PI3K (phosphoinositide 3-kinase)/Akt pathway, three salient molecular features of myostatin action in skeletal muscles. Antagonists to myostatin prevented C26 CM-induced wasting in muscle cell cultures, further confirming that tumoral myostatin may be a key contributor in the pathogenesis of cancer cachexia. Finally, we show that treatment with C26 CM induced the autophagy-lysosome pathway and reduced the number of mitochondria in myotubes. These two previously unreported observations were recapitulated in skeletal muscles collected from C26 tumour-bearing mice.
Lokireddy S, Wijesoma IW, Bonala S et al., Biochem J. 2012 Aug 15;446(1):23-36. doi: 10.1042/BJ20112024.
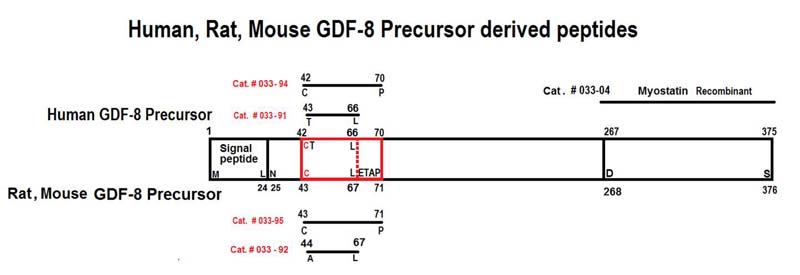
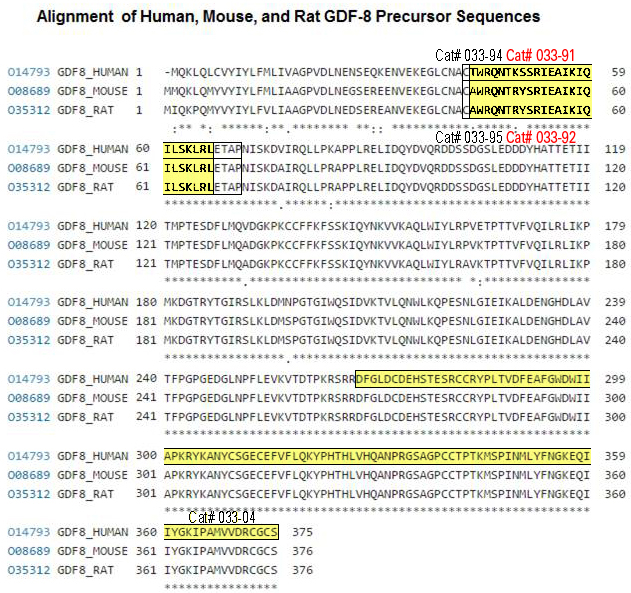
|
|
|
trp-his
%Myostatin%
|
|
|


