|
|
|
sTWEAK |
ELISA and its derived peptides for Obesity, Diabetes and Cardiovascular diseases |
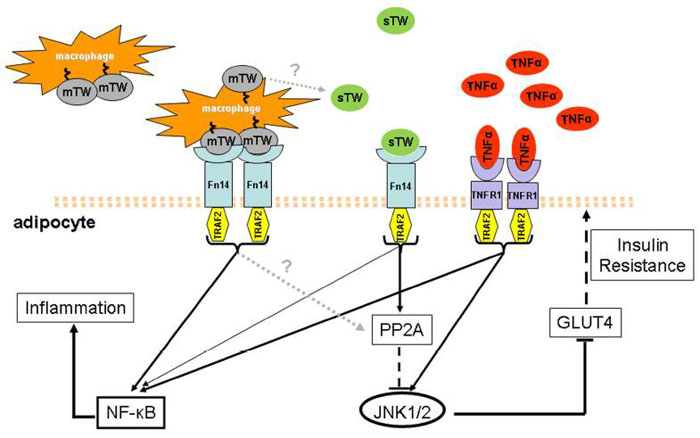
Figure from : Front Immunol. 2013 Dec 30;4:488. eCollection 2013.
|
|
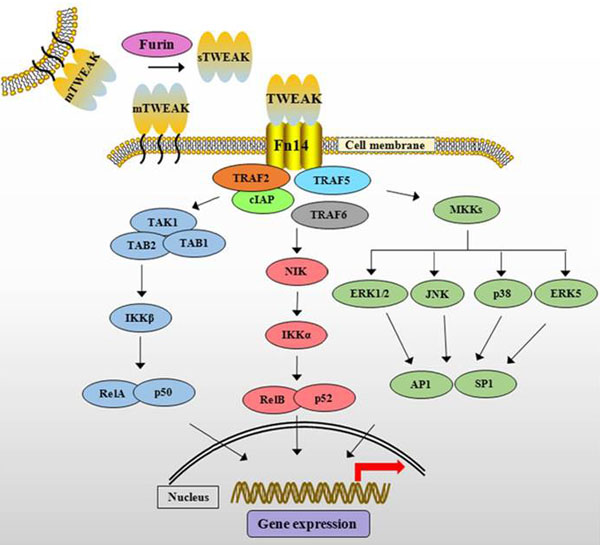
TWEAK cytokine is initially synthesized as membrane-anchored protein (mTWEAK). mTWEAK is then cleaved by furin into soluble form of TWEAK (sTWEAK). Binding of trimeric mTWEAK or sTWEAK to Fn14 receptor leads to the recruitment of cIAPs and various TRAFs resulting in the activation of multiple downstream signaling cascades.
Figure from : Front Immunol. 2014 Jan 27;5:18 |
Skeletal muscle is responsible for the majority of glucose disposal in body. Impairment in skeletal muscle glucose handling capacity leads to the state of insulin resistance. The TNF-like weak inducer of apoptosis (TWEAK) cytokine has now emerged as a major regulator of skeletal muscle mass and function. However, the role of TWEAK in skeletal muscle metabolic function remains less understood. Here, we demonstrate that with progressive age, skeletal muscle-specific TWEAK-transgenic (TWEAK-Tg) mice gain increased body weight (~16%) and fat mass (~64%) and show glucose intolerance and insulin insensitivity. TWEAK-Tg mice also exhibit adipocyte hypertrophy in the epididymal fat. Oxygen uptake, voluntary physical activity, and exercise capacity were significantly reduced in TWEAK-Tg mice compared with controls. Overexpression of TWEAK inhibited (~31%) 5' AMP-activated protein kinase (AMPK) and reduced (~31%) the levels of glucose transporter type 4 (GLUT4) without affecting the Akt pathway. TWEAK also inhibited insulin-stimulated glucose uptake (~32%) and repressed the levels of GLUT4 (~50%) in cultured myotubes from C57BL6 mice. TWEAK represses the levels of Krüppel-like factor 15; myocyte enhancer factor 2, and peroxisome proliferator-activated receptor-γ coactivator-1α, which are required for the activation of the GLUT4 locus. Collectively our study demonstrates that elevated levels of TWEAK in skeletal muscle cause metabolic abnormalities. Inhibition of TWEAK could be a potential approach to prevent weight gain and type 2 diabetes.-Sato, S., Ogura, Y., Tajrishi, M. M., Kumar, A. Elevated levels of TWEAKin skeletalmuscle promote visceral obesity, insulin resistance, and metabolic dysfunction.
Sato S, Ogura Y, Tajrishi MM, Kumar A. FASEB J. 2014 Dec 2. pii: fj.14-260703. [Epub ahead of print]
AIM: To evaluate the inflammatory axis mediated by tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its scavenger receptor CD163 during pregnancy and their influence on insulin sensitivity in normal pregnancy and in Gestational Diabetes Mellitus (GDM).
METHODS: 137 women with one singleton pregnancy, 71 with normal glucose tolerance (NGT) and 66 with GDM were studied. Glucose metabolism was assessed by oral glucose tolerance test. Serum concentrations of soluble TWEAK (sTWEAK) and CD163 (sCD163) and insulin resistance (HOMA-IR index) were determined in maternal blood drawn at recruitment, in the early third trimester. Offspring weight and height were assessed at birth .
RESULTS: Women with GDM had lower circulating sTWEAK concentrations than control NGT group (237.8 (192.1-301.0) pg/ml vs. 277.2 (206.4-355.7) pg/ml; p=0.013). sTWEAK was negatively associated with the presence of GDM (r=-0.212; p=0.013), HOMA-IR index (r=-0.197; p=0.021) and ponderal index of the new-born (r=-0.196; p=0.025), but positively with HDL-cholesterol (r=0.283; p=0.001). In multiple regression analysis, sTWEAK concentration emerged as one of the main predictors of insulin resistance, along with BMI, triglycerides and low concentrations of HDL-cholesterol (R2 =0.486; p<0.001). No relationship was found between HOMA-IR index and sCD163 or sCD163/sTWEAK ratio.
CONCLUSIONS: sTWEAK concentrations are lower in GDM patients compared with healthy pregnant women and low concentrations of sTWEAK are associated with insulin resistance. These findings suggest that insulin resistance during pregnancy is closely linked to inflammatory imbalance and sTWEAK may represent a new candidate associated with GDM.
Simón-Muela I, Llauradó G, Chacón MR et al., Eur J Clin Invest. 2014 Dec 1. doi: 10.1111/eci.12375. [Epub ahead of print]
Obesity and type 2 diabetes (T2D) are associated with chronic low-grade inflammation. Mounting evidence suggests the involvement of an inflammatory switch in adipose tissue, both in mature adipocytes and immune-competent cells from the stromal vascular compartment, in the progression of obesity and insulin resistance. Several inflammatory cytokines secreted by obese adipose tissue, including TNFα and IL-6 have been described as hallmark molecules involved in this process, impairing insulin signaling in insulin-responsive organs. An increasing number of new molecules affecting the local and systemic inflammatory imbalance in obesity and T2D have been identified. In this complex condition, some molecules may exhibit opposing actions, depending on the cell type and on systemic or local influences. Tumor necrosis factor weak inducer of apoptosis (TWEAK), a cytokine of the tumor necrosis (TNF) superfamily, is gaining attention as an important player in chronic inflammatory diseases. TWEAK can exist as a full-length membrane-associated (mTWEAK) form and as a soluble (sTWEAK) form and, by acting through its cognate receptor Fn14, can control many cellular activities including proliferation, migration, differentiation, apoptosis, angiogenesis, and inflammation. Notably, sTWEAK has been proposed as a biomarker of cardiovascular diseases. Here, we will review the recent findings relating to TWEAK and its receptor within the context of obesity and the associated disorder T2D.
Vendrell J, Chacón MR. Front Immunol. 2013 Dec 30;4:488. eCollection 2013.
CONTEXT: Soluble TNF-like weak inducer of apoptosis (sTWEAK) is generated by the intracellular proteolytic cleavage of full-length membrane-bound TNF-like weak inducer of apoptosis (mTWEAK). sTWEAK levels are reduced in diseases with an inflammatory component. Additionally, sTWEAK hampers TNFα activity in human cells.
OBJECTIVES: The objectives of the study were as follows: 1) to determine circulating sTWEAK in severe obesity and after bariatric surgery; 2) to study m/sTWEAK and its receptor fibroblast growth factor-inducible 14 (Fn14) protein expression in sc adipose tissue (SAT) of severely obese subjects, in SAT stromal vascular fraction (SVF), and isolated adipocytes and in human monocyte-derived macrophages; and 3) to explore, on human adipocytes, the sTWEAK effect on TNFα proinflammatory activity.
DESIGN: sTWEAK levels were measured in cohort 1: severely obese subjects (n = 23) and a control group (n = 35); and in cohort 2: (n = 23) severely obese subjects before and after surgery. The m/sTWEAK and Fn14 expressions were determined in SAT biopsies, SVF, and isolated adipocytes from severely obese and control subjects and in human monocyte-derived macrophages. In human primary cultured adipocytes, sTWEAK pretreated and TNFα challenged, IL-6, IL-8, and adiponectin protein and gene expressions were determined and nuclear factor-κ B and MAPK signaling analyzed.
RESULTS: sTWEAK levels were reduced in severely obese subjects. After surgery, sTWEAK levels rose in 69% of patients. mTWEAK protein expression was increased in SAT and SVF of severely obese subjects, whereas Fn14 was up-regulated in isolated adipocytes. M2 human monocyte-derived macrophages overexpress mTWEAK. In human adipocytes, sTWEAK down-regulates TNFα cytokine production by hampering TNFα intracellular signaling events.
CONCLUSION: The decrease of sTWEAK in severely obese patients may favor the proinflammatory activity elicited by TNFα.
Maymó-Masip E, Fernández-Veledo S, Garcia España A et al., J Clin Endocrinol Metab. 2013 Aug;98(8):E1323-33. doi: 10.1210/jc.2012-4177. Epub 2013 Jun 19.
Cardiovascular diseases (CVD) are the first cause of mortality in Western countries. CVD include several pathologies such as coronary heart disease, stroke or cerebrovascular accident, congestive heart failure, peripheral arterial disease, and aortic aneurysm, among others. Interaction between members of the tumor necrosis factor (TNF) superfamily and their receptors elicits several biological actions that could participate in CVD. TNF-like weak inducer of apoptosis (TWEAK) and its functional receptor and fibroblast growth factor-inducible molecule 14 (Fn14) are two proteins belonging to the TNF superfamily that activate NF-κB by both canonical and non-canonical pathways and regulate several cell functions such as proliferation, migration, differentiation, cell death, inflammation, and angiogenesis. TWEAK/Fn14 axis plays a beneficial role in tissue repair after acute injury. However, persistent TWEAK/Fn14 activation mediated by blocking experiments or overexpression experiments in animal models has shown an important role of this axis in the pathological remodeling underlying CVD. In this review, we summarize the role of TWEAK/Fn14 pathway in the development of CVD, focusing on atherosclerosis and stroke and the molecular mechanisms by which TWEAK/Fn14 interaction participates in these pathologies. We also review the role of the soluble form of TWEAK as a biomarker for the diagnosis and prognosis of CVD. Finally, we highlight the results obtained with other members of the TNF superfamily that also activate canonical and non-canonical NF-κB pathway
Blanco-Colio LM, Front Immunol. 2014 Jan 20;5:3. eCollection 2014.
Peritoneal dialysis (PD) is complicated by peritonitis episodes that cause loss of mesothelium and eventually sclerosing peritonitis. An improved understanding of the molecular contributors to peritoneal injury and defense may increase the therapeutic armamentarium to optimize peritoneal defenses while minimizing peritoneal injury. There is no information on the expression and function of the cytokine TWEAK and its receptor Fn14 during peritoneal injury. Fn14 expression and soluble TWEAK levels were measured in human PD peritoneal effluent cells or fluids with or without peritonitis. Fn14 expression was also analyzed in peritoneal biopsies from PD patients. Actions of intraperitoneal TWEAK were studied in mice in vivo. sTWEAK levels were increased in peritoneal effluent in PD peritonitis. Effluent sTWEAK levels correlated with the number of peritoneal macrophages (r=0.491, p=0.002). Potential TWEAK targets that express the receptor Fn14 include mesothelial cells and macrophages, as demonstrated by flow cytometry of peritoneal effluents and by analysis of peritoneal biopsies. Peritoneal biopsy Fn14 correlated with mesothelial injury, fibrosis and inflammation, suggesting a potential deleterious effect of TWEAK/Fn14. In this regard, intraperitoneal TWEAK administration to mice promoted peritoneal inflammation characterized by increased peritoneal effluent MCP-1, Fn14 and Gr1+ macrophages, increased mesothelial Fn14, MCP-1 and CCL21 expression and submesothelial tissue macrophage recruitment. Taken together these data suggest that the TWEAK/Fn14 system may promote inflammation and tissue injury during peritonitis and PD.
Sanz AB, Aroeira LS, Bellon T et al., PlosOne. 2014 Mar 5;9(3):e90399. doi: 10.1371/journal.pone.0090399. eCollection 2014.
INTRODUCTION: TNF-like weak inducer of apoptosis (TWEAK) has been proposed as a mediator of inflammation and bone erosion in rheumatoid arthritis (RA). This study aimed to investigate TWEAK and TWEAK receptor (Fn14) expression in synovial tissue from patients with active and inactive rheumatoid arthritis (RA), osteoarthritis (OA) and normal controls and assess soluble (s)TWEAK levels in the synovial fluids from patients with active RA and OA. Effects of sTWEAK on osteoclasts and osteoblasts were investigated in vitro.
METHODS: TWEAK and Fn14 expression were detected in synovial tissues by immunohistochemistry (IHC). Selected tissues were dual labelled with antibodies specific for TWEAK and lineage-selective cell surface markers CD68, Tryptase G, CD22 and CD38. TWEAK mRNA expression was examined in human peripheral blood mononuclear cells (PBMC) sorted on the basis of their expression of CD22. sTWEAK was detected in synovial fluid from OA and RA patients by ELISA. The effect of sTWEAK on PBMC and RAW 264.7 osteoclastogenesis was examined. The effect of sTWEAK on cell surface receptor activator of NF Kappa B Ligand (RANKL) expression by human osteoblasts was determined by flow cytometry.
RESULTS: TWEAK and Fn14 expression were significantly higher in synovial tissue from all patient groups compared to the synovial tissue from control subjects (P < 0.05). TWEAK was significantly higher in active compared with inactive RA tissues (P < 0.05). TWEAK expression co-localised with a subset of CD38+ plasma cells and with CD22+ B-lymphocytes in RA tissues. Abundant TWEAK mRNA expression was detected in normal human CD22+ B cells. Higher levels of sTWEAK were observed in synovial fluids isolated from active RA compared with OA patients. sTWEAK did not stimulate osteoclast formation directly from PBMC, however, sTWEAK induced the surface expression of RANKL by human immature, STRO-1+ osteoblasts.
CONCLUSIONS: The expression of TWEAK by CD22+ B cells and CD38+ plasma cells in RA synovium represents a novel potential pathogenic pathway. High levels of sTWEAK in active RA synovial fluid and of TWEAK and Fn14 in active RA tissue, together with the effect of TWEAK to induce osteoblastic RANKL expression, is consistent with TWEAK/Fn14 signalling being important in the pathogenesis of inflammation and bone erosion in RA.
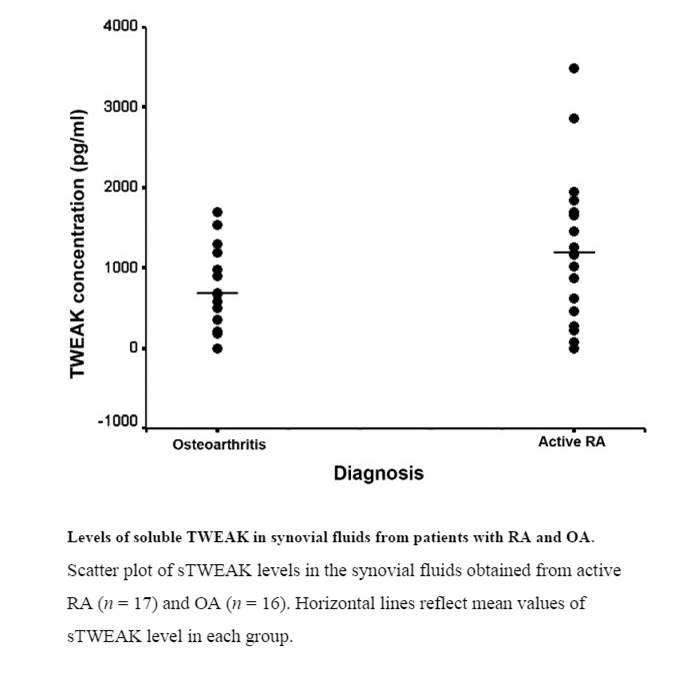
Dharmapatni AA, Smith MD, Crotti TN et al., Arthritis Res Ther. 2011 Mar 24;13(2):R51. doi: 10.1186/ar3294.

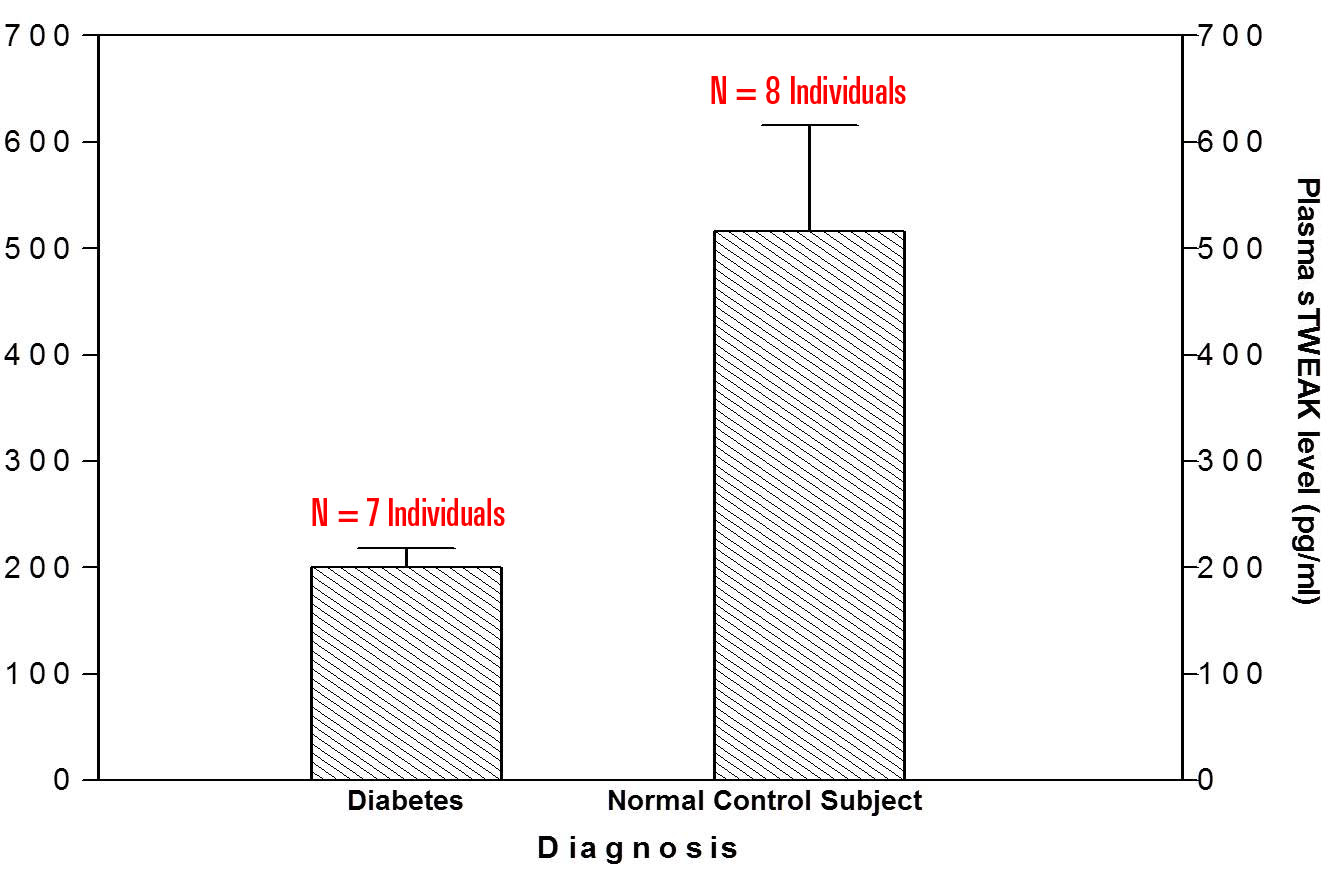

|
|
|
TRAIL
%054-85%;054-78;054-79;054-80
|
|
|


