Pigment epithelium-derived factor (PEDF) is an antiinflammatory protein that circulates at high levels in the metabolic syndrome. Metabolic studies of PEDF knockout (KO) mice were conducted to investigate the relationship between PEDF, inflammatory markers, and metabolic homeostasis. Male PEDF KO mice demonstrated a phenotype consisting of increased adiposity, glucose intolerance, and elevated serum levels of metabolites associated with the metabolic syndrome. Genome expression analysis revealed an increase in IL-1β signaling in the livers of PEDF KO mice that was accompanied by impaired IRS and Akt signaling. In human hepatocytes, PEDF blocked the effects of an IL-1β challenge by suppressing activation of the inflammatory mediator c-Jun N-terminal kinase while restoring Akt signaling. RNA interference of PEDF in human hepatocytes was permissive for c-Jun N-terminal kinase activation and decreased Akt signaling. A metabolomics profile identified elevated circulating levels of tricarboxyclic acid cycle intermediates including succinate, an inducer of IL-1β, in PEDF KO mice. Succinate-dependent IL-1β expression was blocked by PEDF in PEDF KO, but not wild-type hepatocytes. In vivo, PEDF restoration reduced hyperglycemia and improved hepatic insulin signaling in PEDF KO mice. These findings identify elevated PEDF as a homeostatic mechanism in the human metabolic syndrome.
Gattu AK, Birkenfeld AL, Iwakiri Y et al., Endocrinology. 2014 Apr;155(4):1373-85. doi: 10.1210/en.2013-1785. Epub 2014 Jan 23.
Oxidative stress and inflammation in the adipose tissues contribute to the metabolic syndrome. Pigment epithelium-derived factor (PEDF) inhibits vascular inflammation through its anti-oxidative properties. However, it remains unclear whether PEDF could suppress adipocyte inflammation. We investigated the effects of long-term administration or suppression of PEDF on adipocyte inflammation and metabolic derangements in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, an animal model of type 2 diabetes with insulin resistance. Circulating and adipose tissue PEDF levels were increased as OLETF rats became more obese and insulin resistant. Long-term administration of PEDF improves metabolic parameters, ameliorates dysregulation of adipocytokines, and suppresses NADPH oxidase-induced oxidative stress and macrophage infiltration in the adipose tissues of OLETF rats, whereas these variables are exacerbated by the knockdown of PEDF by administering siRNAs. Our study suggests that PEDF could improve metabolic derangements by suppressing the inflammatory and oxidative reactions in adipose tissues of OLETF rats. PEDF levels may be elevated as a countersystem against obesity-related metabolic derangements.
Matsui T, Nishino Y, Ojima A, et al., Am J Pathol. 2014 Apr;184(4):1094-103. doi: 10.1016/j.ajpath.2013.12.032. Epub 2014 Feb 13.
Pigment epithelium-derived factor (PEDF) has been shown previously to prevent liver fibrosis and hepatic stellate cell (HSC) activation. By investigating the functional domains in PEDF, we identified a 34-mer peptide (residues Asp44-Asn77) that harbors the same function as the full-length PEDF protein. Not only did the 34-mer suppress the development of fibrosis in carbon tetrachloride (CCl4)-treated mouse liver but it also upregulated peroxisome proliferator-activated receptor-gamma (PPARγ) expression in HSCs in vivo. Platelet-derived growth factor (PDGF) plays a crucial role on the process of HSC activation in response to liver damage. The 34-mer suppressed PDGF-induced cell proliferation and expression of myofibroblastic marker proteins in primary rat HSC culture, increased the levels of PPARγ mRNA and protein in a dose-dependent manner and markedly reduced the level of active β-catenin protein, an HSC activating factor, in HSC-T6 cells. Similarly, IWR-1, an inhibitor of the Wnt response, displayed the same effect as the 34-mer in preventing HSC-T6 activation. The Wnt signaling-mediated PPARγ suppression was abolished by both the IWR-1 inhibitor and a small interfering RNA (siRNA) targeting β-catenin and the Wnt coreceptor, LRP6. Both PEDF and the 34-mer down-regulated PDGF receptor-α/β expression and blocked the PDGF-induced phosphorylation of Akt and ERK. Moreover, the inhibitory effect on PDGF receptor expression was abolished by PPARγ antagonists and PPARγ siRNA. Our observations indicate that the PEDF-derived 34-mer peptide can mimic PEDF in attenuating HSC activation. Investigation of this 34-mer peptide led to the identification of a signaling mechanism involving PPARγ induction, suppression of Wnt/β-catenin signaling and down-regulation of the PDGF receptor-α/β.
Tsai TH, Shih SC, Ho TC et al., PLoS One. 2014 Apr 24;9(4):e95443. doi: 10.1371/journal.pone.0095443. eCollection 2014.
Objective:
Physiological hypertrophy is featured by the hypertrophy of pre-existing cardiomyocytes and the formation of new cardiomyocytes. C-kit positive cardiac progenitor cells increased their numbers in exercise-induced physiological hypertrophy. However, the participation of Sca-1 positive cells in the physiological adaptation of the heart to exercise training is unclear.
Methods:
Physiological hypertrophy was induced by swimming and the mRNA levels of GATA binding protein 4 (GATA4), atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), endogenous hepatocyte growth factor (HGF), and insulin like growth factor-1 (IGF-1) from the whole heart were determined by real-time polymerase chain reactions (RT-PCRs) analysis. Immunofluorescent staining was used to compare the number of C-kit and Sca-1 positive cardiac progenitor cells. In addition, mRNA levels of C-kit and Sca-1 in left ventricle (LV), right ventricle (RV), and outflow tract (OFT) were determined in mice swimming for 7, 14, and 21 days by RT-PCRs.
Results:
The ratio of heart weight (HW) to body weight and HW to tibia length and the mRNA level of GATA4 were increased while mRNA levels of ANP and BNP remained unchanged. C-kit and Sca-1 positive cardiac progenitor cells were activated by swimming training. An increased endogenous production of HGF and IGF was observed at least at the mRNA level. Swimming induced a significant up-regulation of C-kit in LV of mice swimming for 1, 2 and 3 weeks and in RV of mice swimming for 3 weeks. Sca-1 positive cardiac progenitor cells were increased in LV and OFT in mice swimming for 3 weeks.
Conclusion: This study presents that swimming-induced physiological hypertrophy initiates activation of cardiac progenitor cells.
Junjie Xiao, Tianzhao Xu, Jin Li, et al., Int J Clin Exp Pathol. 2014 Jan 15;7(2):663-9. eCollection 2014.
BACKGROUND:
Accurate risk prediction is important for an adequate management of heart failure (HF) patients. We assessed the incremental prognostic ability of a multi-biomarker approach in advanced HF.
METHODS:
In 349 patients with advanced HF (median 75 years, 66% male) we investigated the incremental prognostic value of 12 novel biomarkers involved in different pathophysiological pathways including inflammation, immunological activation, oxidative stress, cell growth, remodeling, angiogenesis and apoptosis.
RESULTS:
During a median follow-up of 4.9 years 55.9% of patients died. Using multivariable Cox regression and bootstrap variable-selection age, chronic obstructive pulmonary disease, N-terminal pro-B-type natriuretic peptide (NT-proBNP) and the following 5 novel biomarkers were retained in the best mortality prediction model: the chemokine fractalkine, the angiogenic and mitogenic hepatocyte growth factor (HGF), the growth differentiation factor 15 (GDF-15) influencing cardiac remodeling and apoptosis, and the 2 pro-apoptotic molecules soluble apoptosis-stimulating fragment (sFAS) and soluble tumor necrosis factor-related apoptosis-inducing ligand (sTRAIL). This multi-biomarker score had strong discriminatory power for 5-year mortality (area under the Receiver Operating Characteristic curve [AUC]=0.81) and improved risk prediction beyond the prognostic power of a comprehensive conventional risk score including known clinical predictors and NT-proBNP (AUC=0.77). Net reclassification confirmed a significant improvement of individual risk prediction (p=0.003).
CONCLUSIONS:
Risk prediction by a multi-biomarker score is superior to a conventional risk score including clinical parameters and NT-proBNP. Additional predictive information from different biological pathways reflects the multisystemic character of HF.
Richter B, Koller L, Hohensinner PJ et al., Int J Cardiol. 2013 Sep 30;168(2):1251-7. doi: 10.1016/j.ijcard.2012.11.052. Epub 2012 Dec 4.
Background:
Hepatocyte growth factor (HGF) concentration increases in the first few hours of myocardial infarction (MI).
Aim:
(1) To illustrate human HGF (hHGF) plasma concentration during the first 24 h of ST segment elevation myocardial infarction (STEMI); (2) To estimate the odds ratio of STEMI in the context of hHGF measurements; (3) To describe the normal concentration of hHGF in healthy subjects.
Methods:
The study groups consisted of 73 STEMI patients and 11 healthy volunteers. In all the patients, we took blood samples for hHGF twice, i.e. on admission to hospital and 24 h later.
Results:
The median value of hHGF in healthy volunteers was 666 pg/mL (576; 760 pg/mL). In STEMI, the highest values of hHGF were observed in the first measurement. An increase of 1 pg/mL in hHGF level increased STEMI odds ratio by 0.2%.
Conclusions:
In acute MI, of the known biomarkers, hHGF rises the earliest and very promptly returns to normal values. Key words: myocardial infarction, STEMI, biomarkers, hepatocyte growth factor.
Kardiologia Polska (Kardiol Pol ) 2013; 71, 8: 827–831; DOI: 10.5603/KP.2013.0194 ISSN 0022
Proteins secreted by skeletal muscle, so called myokines, have been shown to affect muscle physiology and additionally exert systemic effects on other tissues and organs. Although recent profiling studies have identified numerous myokines, the amount of overlap from these studies indicates that the secretome of skeletal muscle is still incompletely characterized. One limitation of the models used is the lack of contraction, a central characteristic of muscle cells. Here we aimed to characterize the secretome of primary human myotubes by cytokine antibody arrays and to identify myokines regulated by contraction, which was induced by electrical pulse stimulation (EPS). In this study, we validated the regulation and release of two selected myokines, namely pigment epithelium derived factor (PEDF) and dipeptidyl peptidase 4 (DPP4), which were recently described as adipokines. This study reveals that both factors, DPP4 and PEDF, are secreted by primary human myotubes. PEDF is a contraction-regulated myokine, although PEDF serum levels from healthy young men decrease after 60 min cycling at VO2max of 70%. Most interestingly, we identified 52 novel myokines which have not been described before to be secreted by skeletal muscle cells. For 48 myokines we show that their release is regulated by contractile activity. This profiling study of the human skeletal muscle secretome expands the number of myokines, identifies novel contraction-regulated myokines and underlines the overlap between proteins which are adipokines as well as myokines.
Silja Raschke, Kristin Eckardt, Kirsten Bjørklund Holven, et al, PLoS One. 2013; 8(4): e62008. Published online 2013 April 24. doi: 10.1371/journal.pone.006200
BACKGROUND:
Pigment epithelium-derived factor (PEDF) has been proved to be closely correlated with metabolic syndrome (MetS) and its components that are all risk factors of cardiovascular disease and may play a protective role against vascular injury and atherosclerosis. The present study was designed to investigate the relationship between serum PEDF and coronary artery disease (CAD).
METHODS:
A total of 312 consecutive in-patients (including 228 with CAD and 197 with MetS) who underwent coronary angiography were enrolled. Serum PEDF was measured by sandwich enzyme immunoassay and used to carry out multivariate stepwise regression analysis to assess correlation with patient demographic and clinical parameters. Multiple logistic regression analysis was performed to identify factors independently correlated with CAD.
RESULTS:
Patients with MetS had significantly higher levels of serum PEDF than non-MetS subjects (11.1(8.2, 14.2) vs. 10.1(7.6, 12.4) μg/mL; P < 0.05). Patients with CAD also had significantly higher serum PEDF than non-CAD subjects (11.0(8.1, 14.2) vs. 10.3(8.1, 12.8) μg/mL; P < 0.05). Triglyceride (TG), C-reactive protein (CRP), estimated glomerular filtration rate (eGFR), and hypoglycemic therapy were independently correlated with serum PEDF levels, and serum PEDF was independently positively correlated with CAD.
CONCLUSIONS:
Serum PEDF levels are independently positively associated with CAD in a Chinese population. Elevated PEDF may act as a protective response against vascular damage and subsequent CAD.
Feifei Wang, Xiaojing Ma, Mi Zhou et al., Cardiovasc Diabetol. 2013; 12: 56. Published online 2013 April 1. doi: 10.1186/1475-2840-12-56
We have previously found that serum level of pigment epithelium-derived factor (PEDF) is increased in proportion to the accumulation of the number of components of metabolic syndrome in general population. However, the link between PEDF and insulin resistance in hypertensive patients remains unclear. We investigated here whether serum PEDF level was associated with insulin resistance in hypertensive patients. Ninety-seven consecutive outpatients with essential hypertension underwent a complete history and physical examination, determination of blood chemistries, including serum PEDF. In multiple regression analyses, BMI, age (inversely) and PEDF were independently correlated with homeostasis model assessment of insulin resistance (HOMA-IR). When age- and sex-adjusted PEDF levels were stratified by HOMA-IR tertiles, a linear and significant trend was observed. These results demonstrated that serum level of PEDF was an independent determinant of HOMA-IR in patients with essential hypertension. PEDF may play a role in insulin resistance in hypertensive patients.
Nakamura K, Yamagishi S, Adachi H et al., International Journal of Cardiology�Volume 143, Issue 1 , Pages 96-98, 6 August 2010
Objective:
Pigment epithelium-derived factor (PEDF) is a multifunctional protein with neurotrophic and anti-angiogenic properties. More recently it became evident that PEDF is upregulated in patients with type 2 diabetes and also contributes to insulin resistance in mice. During characterization of the secretome of in vitro differentiated human adipocytes by two-dimensional polyacrylamide gel electrophoresis and matrix-assisted laser desorption/ionization-MS, we found that PEDF is one of the most abundant proteins released by adipocytes. The aim of this study was to investigate the regulation and autocrine function of PEDF in human adipocytes and to determine its paracrine effects on human skeletal muscle cells (hSkMC) and human smooth muscle cells (hSMC).
Methods and Results:
Human primary adipocytes secrete 130 ng ml−1 PEDF over 24 h from 1 million cells, which is extremely high as compared with adiponectin, interleukin-6 (IL-6) or IL-8. This release of PEDF is significantly higher than from other primary cells, such as adipose-tissue located macrophages (50-times), hSkMC and hSMC (5-times). PEDF protein expression significantly increases during adipogenesis, which is paralleled by increased PEDF secretion. Furthermore, tumor necrosis factor-α and hypoxia significantly downregulate PEDF protein levels. PEDF secretion was significantly reduced by troglitazone and hypoxia and significantly increased by insulin. Treatment of adipocytes and hSkMC with PEDF induced insulin resistance in adipocytes, skeletal and smooth muscle cells at the level of insulin-stimulated Akt phosphorylation, which was dose dependent and more prominent in adipocytes. Furthermore, inflammatory nuclear factor-κB (NF-κB) signaling was induced by PEDF. In hSMC, PEDF induced proliferation (1.7-fold) and acutely activated proliferative and inflammatory signaling pathways (NF-κB, p38 mitogen-activated protein kinase and mammalian target of rapamycin).
Conclusion:
PEDF is one of the most abundant adipokines and its secretion is inversely regulated by insulin and hypoxia. PEDF induces insulin resistance in adipocytes and hSkMC and leads to inflammatory signaling in hSMC. Because of these diverse actions, PEDF is a key adipokine, which could have an important role in diabetes and obesity-related disorders.
Famulla S, Lamers D, S. Hartwig, W et al., Int J Obes (Lond). 35, 762-772 (June 2011) | doi:10.1038/ijo.2010.212
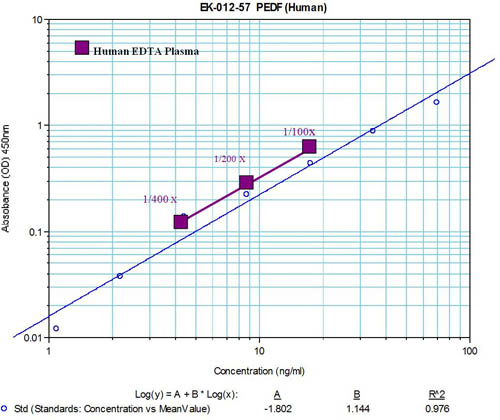
Minimum detection level: 1.08 ng/ml
Range: 1-70 ng/ml |
|
|
Note: * Human plasma samples (collected with EDTA) were
denatured with 8M Urea and diluted with assay buffer at 1:200 dilution
** Varying concentrations of PEDF were spiked into diluted human plasma. Spiked samples were then divided into three groups. This data represents measured concentrations after subtraction of the un-spiked plasma. |
Human plasma samples (collected with EDTA) after treated with urea were diluted with assay buffer at 1:50.
Diluted human plasma was assayed with EK-012-57. Data is presented as mean ± S.E. for each group of samples.
Â