|
|
|
Bioactive Fractalkine |
Peptides and ELISA kit for the neuroinflammatory and metabolic disease |
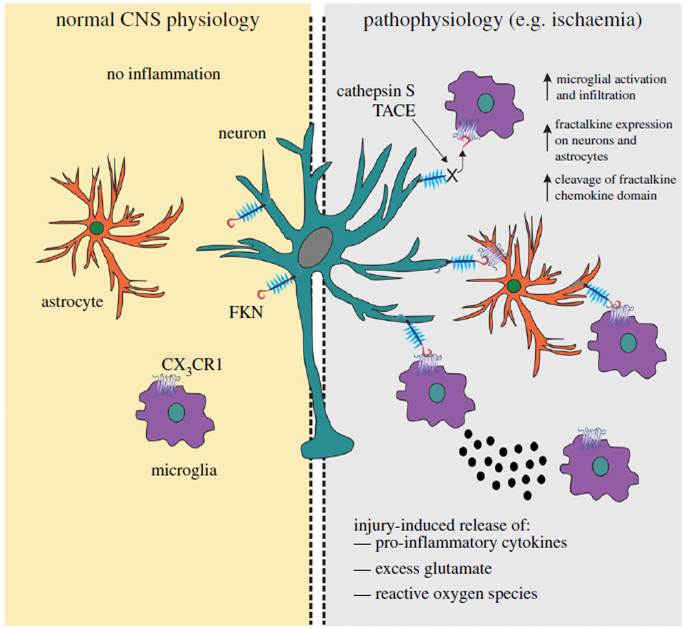
In the uninjured brain uncder normal physiological conditions, fractalkine (FKN) is largely expressed on neeurons and CX3CR1 on microglial cells. FKN sequesters microglia in a quiescent “inactive” state. Astrocytes are relatively devoid of FKN and CX3CR1 protein expression. TNF-α-converting enzyme (TACE) and cathepsin S, released during injury. Upregulated levels of FKN can attract microglia to the site of inflammation, where they become activated and release pro-inflammatory mediatory mediators such as cytokines, reactive oxygen species and glutamate. Astrocytes can also express FKN following an inflammatory insult and thus can communicate with both neurons and microglia via CX3CR1. The increased expression of FKN should have a net anti-inflammatory action and serve to limit inflammation in favor of functional recovery of CNS tissues.
Sheridan GK and Murphy KJ. Open Biol. 2013 Dec 18;3(12):130181. doi: 10.1098/rsob.130181.
Chemokines are molecules able to induce chemotaxis of monocytes, neutrophils, eosinophils, lymphocytes and fibroblasts. The complex chemokine acts in many physiological and pathological phenomena, including those occurring in the articular cartilage. To date, chemokine CX3CL1 (fractalkine) is the only member of the CX3C class of chemokines with well-documented roles in endothelial cells. CX3CL1 is a unique chemokine that combines properties of chemoattractant and adhesion molecule. The main roles of CX3CL1 include promotion of leukocyte binding and adhesion as well as activation of the target cells. The soluble chemokine domain of CX3CL1 is chemotactic for T cells and monocytes. CX3CL1 acts via its receptor, CX3CR1, which belongs to a family of G protein-coupled receptors. Stimulation of CX3CR1 activates both CX3CL1-dependent and integrin-dependent migrations of cells with synergistically augmented adhesion. Genetic polymorphisms of CX3CR1 may significantly modify the biological roles of CX3CL1, especially in pathologic conditions. Osteoarthritis (OA) is the most common joint disease, affecting approximately 7-8 % of the general population. Development of OA is largely driven by low-grade local background inflammation involving chemokines. The importance of CX3CL1/CX3CR1 signalling in the pathophysiology of OA is still under investigation. This paper, based on a review of the literature, updates and summarises the current knowledge about CX3CL1/CX3CR1 in OA and indicates possible interactions with a potential for therapeutic targeting.
Wojdasiewicz P, Poniatowski LA, Kotela A et al., Arch Immunol Ther Exp (Warsz). 2014 Feb 21. [Epub ahead of print]
Endurance exercise is associated with significant improvements in cardio-metabolic risk parameters. A role for myokines has been hypothesized, yet limited information is available about myokines induced by acute endurance exercise in humans. Therefore the aim of the study was to identify novel exercise-induced myokines in humans. To this end, we carried out a one hour one-legged acute endurance exercise intervention in 12 male subjects and a 12 week exercise training intervention in 18 male subjects. Muscle biopsies were taken before and after acute exercise or exercise training, and were subjected to microarray-based analysis of secreted proteins (secretome). For acute exercise, secretome analysis resulted in a list of 86 putative myokines, which was reduced to 29 by applying a fold-change cut-off of 1.5. Based on that shortlist, a selection of putative myokines was measured in the plasma using ELISA or multiplex assay. From that selection, CX3CL1 (fractalkine) and CCL2 (MCP-1) increased at both mRNA and plasma level. From the known myokines, only IL-6 and FGF21 changed at the mRNA level, whereas none of the known myokines changed at the plasma level. Secretome analysis of exercise training intervention resulted in a list of 69 putative myokines. Comparing putative myokines altered by acute exercise and exercise training revealed a very limited overlap of only 13 genes. In conclusion, this study identified CX3CL1 and CCL2 as myokines that were induced by acute exercise at the gene expression and plasma level and that may be involved in communication between skeletal muscle and other organs.
Catoire M, Mensink M, Kalkhoven E et al., Physiol Genomics. 2014 Feb 11. [Epub ahead of print]
An essential aspect of normal brain function is the bidirectional interaction and communication between neurons and neighbouring glial cells. To this end, the brain has evolved ligand-receptor partnerships that facilitate crosstalk between different cell types. The chemokine, fractalkine (FKN), is expressed on neuronal cells, and its receptor, CX3CR1, is predominantly expressed on microglia. This review focuses on several important functional roles for FKN/CX3CR1 in both health and disease of the central nervous system. It has been posited that FKN is involved in microglial infiltration of the brain during development. Microglia, in turn, are implicated in the developmental synaptic pruning that occurs during brain maturation. The abundance of FKN on mature hippocampal neurons suggests a homeostatic non-inflammatory role in mechanisms of learning and memory. There is substantial evidence describing a role for FKN in hippocampal synaptic plasticity. FKN, on the one hand, appears to prevent excess microglial activation in the absence of injury while promoting activation of microglia and astrocytes during inflammatory episodes. Thus, FKN appears to be neuroprotective in some settings, whereas it contributes to neuronal damage in others. Many progressive neuroinflammatory disorders that are associated with increased microglial activation, such as Alzheimer's disease, show disruption of the FKN/CX3CR1 communication system. Thus, targeting CX3CR1 receptor hyperactivation with specific antagonists in such neuroinflammatory conditions may eventually lead to novel neurotherapeutics.
Sheridan GK, Murphy KJ. Open Biol. 2013 Dec 18;3(12):130181. doi: 10.1098/rsob.130181.
Leukocyte chemotaxis to the area of tissue damage is mediated by chemokines. According to the primary structure, chemokines are divided into four families, fractalkine (CX3CL1) is the only one member of CX3C family and the only membrane-bound chemokine. Fractalkine molecule includes the extracellular N-terminal chemokine domain, mucin-like rod, the transmembrane and the intracellular domains. In membrane-bound state fractalkine has the properties of an adhesion molecule. Chemokine domain of fractalkine (CDF) is released from cell membrane by proteolysis, and this soluble form acts as a chemoattractant for leukocytes expressing fractalkine receptor CX3CR1. Fractalkine is involved in development of a number of pathological processes caused by inflammation, and therefore a search for fractalkine inhibitors is very important. For this purpose we identified several antigenic determinants-the fragments of CDF, and the following peptides were synthesized-P41-52 H-Leu-Glu-Thr-Arg-Gln-His-Arg-Leu-Phe-Cys-Ala-Asp-NH2, P53-60 H-Pro-Lys-Glu-Gln-Trp-Val-Lys-Asp-NH2 and P60-71 H-Asp-Ala-Met-Gln-His-Leu-Asp-Arg-Gln-Ala-Ala-Ala-NH2. The peptide effects on adhesion and migration of human peripheral blood monocytes expressing fractalkine receptors were investigated. In the presence of CDF and P41-52 we observed the increased adhesion and migration of monocytes compared with spontaneous values. Peptides P53-60 and P60-71 significantly inhibited monocyte adhesion and migration stimulated by CDF. Since the chemotactic activity of chemokines was shown to be dependent on their binding to glycosaminoglycans of the cell surface and extracellular matrix, the effect of peptides on the interaction of CDF with heparin was analyzed by ELISA. Peptide P41-52 competed with CDF for heparin binding, while peptides P53-60 and P60-71 had no significant activity.
Kukhtina NB, Aref'eva TI, Ruleva NIu, et al., Bioorg Khim. 2012 Nov-Dec;38(6):660-6.
CX3CL1 chemokine (fractalkine) is highly expressed by vascular smooth muscle cells (VSMCs) in atherosclerotic lesions. Its membrane-bound form promotes cell-cell interactions, whereas the soluble form induces chemotaxis of CX3CR1- expressing leukocytes. We show that the cysteine protease cathepsin S, expressed by VSMCs, is able to cleave membrane-anchored CX3CL1, releasing a 55-kDa fragment to the medium, thus regulating the adhesion of VSMCs and the capture of monocytes to the sites of atherogenesis. Moreover, strong co-localization of cathepsin S and CX3CL1 with a recycling endosome marker Rab11a suggests a processing of CX3CL1 in recycling endosomes during its redistribution to the plasma membrane.
Fonović UP, Jevnikar Z, Kos J. Biol Chem. 2013 Oct;394(10):1349-52. doi: 10.1515/hsz-2013-0189.
OBJECTIVE: Alterations of the chemokine receptor CX3CR1 gene were associated with a reduced risk of myocardial infarction in human and limited atherosclerosis in mice. In this study, we addressed whether CX3CR1 antagonists are potential therapeutic tools to limit acute and chronic inflammatory processes in atherosclerosis.
APPROACH AND RESULTS: Treatment with F1, an amino terminus-modified CX3CR1 ligand endowed with CX3CR1 antagonist activity, reduced the extent of atherosclerotic lesions in both Apoe(-/-) and Ldlr(-/-) proatherogenic mouse models. Macrophage accumulation in the aortic sinus was reduced in F1-treated Apoe(-/-) mice but the macrophage density of the lesions was similar in F1-treated and control mice. Both in vitro and in vivo F1 treatment reduced CX3CR1-dependent inflammatory monocyte adhesion, potentially limiting their recruitment. In addition, F1-treated Apoe(-/-) mice displayed reduced numbers of blood inflammatory monocytes, whereas resident monocyte numbers remained unchanged. Both in vitro and in vivo F1 treatment reduced CX3CR1-dependent inflammatory monocyte survival. Finally, F1 treatment of Apoe(-/-) mice with advanced atherosclerosis led to smaller lesions than untreated mice but without reverting to the initial phenotype.
CONCLUSIONS: The CX3CR1 antagonist F1 is a potent inhibitor of the progression of atherosclerotic lesions by means of its selective impact on inflammatory monocyte functions. Controlling monocyte trafficking and survival may be an alternative or complementary therapy to lipid-lowering drugs classically used in the treatment of atherosclerosis.
Poupel L, Boissonnas A, Hermand P et al., Arterioscler Thromb Vasc Biol. 2013 Oct;33(10):2297-305. doi: 10.1161/ATVBAHA.112.300930. Epub 2013 Jul 25.
AIMS/HYPOTHESIS: Pioglitazone (PIO) is a peroxisome proliferator-activated receptor (PPAR)γ agonist insulin-sensitiser with anti-inflammatory and anti-atherosclerotic effects. Our objective was to evaluate the effect of low-dose PIO (15 mg/day) on glucose metabolism and inflammatory state in obese individuals with type 2 diabetes.
METHODS: A randomised, double-blind, placebo-controlled, mechanistic trial was conducted on 29 patients with type 2 diabetes treated with metformin and/or sulfonylurea. They were randomised to receive PIO or placebo (PLC) for 6 months, in a 1:1 ratio. Participants were allocated to interventions by central office. All study participants, investigators and personnel performing measurements were blinded to group assignment. At baseline and after 6 months patients underwent: (1) OGTT; (2) muscle biopsy to evaluate expression of TNF-α, tissue inhibitor of metalloproteases 3 (TIMP-3) levels, TNF-α converting enzyme (TACE) expression and enzymatic activity; (3) euglycaemic-hyperinsulinaemic clamp; (4) measurement of plasma high-sensitivity C-reactive protein (hsCRP), plasminogen activator inhibitor type-1 (PAI-1), TNF-α, IL-6, monocyte chemotactic protein-1 (MCP-1), adiponectin and fractalkine (FRK). The interventions were PIO 15 mg/day vs placebo and the main outcomes measured were absolute changes in whole-body insulin sensitivity, insulin secretion and inflammatory state.
RESULTS: Fifteen participants were randomized to receive PIO and 14 participants were randomized to receive PLC. Eleven participants completed the study in the PIO group and nine participants completed the study in the PLC group and were analysed. Fasting plasma glucose and HbA1c decreased modestly (p < 0.05) after PIO and did not change after PLC. M/I (insulin-stimulated whole-body glucose disposal), adipose tissue insulin resistance (IR) index, insulin secretion/IR (disposition) index and insulinogenic index improved significantly after PIO, but not after PLC. Circulating MCP-1, IL-6, FRK, hsCRP and PAI-1 levels decreased in PIO- as compared with PLC-treated patients, while TNF-α did not change. TNF-α protein expression and TACE enzymatic activity in muscle were significantly reduced by PIO but not PLC. Adiponectin levels increased significantly after PIO as compared with PLC treatment. Given that the mean TACE enzymatic activity level at baseline in the PIO group was 0.29 ± 0.07 (fluorescence units [FU]), and at end of study decreased to 0.05 vs 0.14 in the PLC group, the power to reject the null hypothesis that the population means of the PIO and PLC groups are equal after 6 months is greater than 0.80. Given that M/I was 2.41 ± 0.35 μmol kg(-1) min(-1) (pmol/l)(-1) at baseline and increased by 0.55 in the PIO and 0.17 in the PLC groups, the power to reject the null hypothesis that the population means of the PIO and PLC groups are equal after 6 months is greater than 0.85. The type I error probability associated with this test of this null hypothesis is 0.05. No serious adverse events occurred in either group.
CONCLUSIONS/INTERPRETATION: Low-dose PIO (15 mg/day) improves glycaemic control, beta cell function and inflammatory state in obese patients with type 2 diabetes.
Tripathy D, Daniele G, Fiorentino TV et al., Diabetologia. 2013 Oct;56(10):2153-63. doi: 10.1007/s00125-013-2976-z. Epub 2013 Jun 30.
Renal biopsy is the gold standard for confirmation of disease severity and diagnosis of glomerulonephritis (GN), but its procedure is invasive with a risk of complications. Thus, a non-invasive monitoring method is desirable especially in pediatric patients. Fractalkine and monocyte chemoattractant protein-1 (MCP-1) are proinflammatory chemokines, and both have been reported to be involved in the pathogenesis of immunocomplex-mediated GN. Recently, it has been reported that urinary fractalkine and MCP-1 may serve as possible predictors of disease activity of adult patients with GN. We, therefore, examined whether urinary levels of fractalkine and MCP-1 correlate with clinical and histologic parameters. Twenty-six consecutive children with various types of GN were enrolled in this study, including lupus nephritis, IgA nephropathy, membranous nephropathy, acute GN, and thin basement membrane disease (served as a non-inflammatory control). Urinary fractalkine and MCP-1 concentrations in the first morning urine samples obtained at the time of renal biopsy were measured by enzyme-linked immunosorbent assay, and standardized for urinary creatinine concentrations. Urinary fractalkine concentration differed significantly among the disease categories. Urinary concentrations of fractalkine and MCP-1 showed a significant positive correlation with the degree of occult blood in urine and a significant inverse correlation with the estimated glomerular filtration rate. Furthermore, the urinary MCP-1 concentration was significantly correlated with histological chronicity indices in patients with lupus nephritis and IgA nephropathy. Measurement of urinary fractalkine and MCP-1 concentrations may be useful as a non-invasive method for predicting the disease activity of GN in children.
Aizawa T, Imaizumi T, Tsuruga K et al., Tohoku J Exp Med. 2013;231(4):265-70.
Here, we demonstrate that the fractalkine (FKN)/CX3CR1 system represents a regulatory mechanism for pancreatic islet β cell function and insulin secretion. CX3CR1 knockout (KO) mice exhibited a marked defect in glucose and GLP1-stimulated insulin secretion, and this defect was also observed in vitro in isolated islets from CX3CR1 KO mice. In vivo administration of FKN improved glucose tolerance with an increase in insulin secretion. In vitro treatment of islets with FKN increased intracellular Ca(2+) and potentiated insulin secretion in both mouse and human islets. The KO islets exhibited reduced expression of a set of genes necessary for the fully functional, differentiated β cell state, whereas treatment of wild-type (WT) islets with FKN led to increased expression of these genes. Lastly, expression of FKN in islets was decreased by aging and high-fat diet/obesity, suggesting that decreased FKN/CX3CR1 signaling could be a mechanism underlying β cell dysfunction in type 2 diabetes
Lee YS, Morinaga H, Kim JJ et al., Cell. 2013 Apr 11;153(2):413-25. doi: 10.1016/j.cell.2013.03.001.
AIMS: Fractalkine (FKN) is a newly identified membrane-bound chemokine; its role in myocardial ischaemia and heart failure is largely unknown. We attempted to investigate the role of FKN in myocardial ischaemia and ischaemia or pressure overload-induced ventricular remodelling and heart failure.
METHODS AND RESULTS: FKN-induced changes of heart failure-related genes in cultured rat cardiac cells and the effect of FKN on cultured cardiomyocyte injury during anoxia/reoxygenation (A/R) were examined. The direct influence of FKN neutralization on heart failure and the potential mechanism was also investigated. In mice with failing hearts, myocardial FKN expression was correlated with the lung weight/body weight ratio, left ventricular fractional shortening, and brain natriuretic peptide expression. In cultured rat cells, exposure to FKN increased natriuretic peptide A expression in cardiomyocytes, matrix metalloproteinase-9 expression in fibroblasts, and intercellular adhesion molecule-1 expression in microvascular endothelial cells. FKN also promoted cardiomyocyte damage during A/R and neutralizing FKN antibody treatment improved heart failure induced by myocardial infarction or pressure overload. Neutralizing FKN or its receptor inhibited the activation of mitogen-activated protein kinases (MAPKs) in hypoxic cardiomyocytes or ischaemic myocardium.
CONCLUSION: FKN promotes myocardial injury and accelerates the progress of heart failure, which is associated with the activation of MAPKs.
Xuan W, Liao Y, Chen B et al., Cardiovasc Res. 2011 Dec 1;92(3):385-93. doi: 10.1093/cvr/cvr221. Epub 2011 Aug 12.
The CX3C chemokine family is composed of only one member, CX3CL1, also known as fractalkine, which in mice is the sole ligand of the G protein-coupled, 7-transmembrane receptor CX3CR1. Unlike classic small peptide chemokines, CX3CL1 is synthesized as a membrane-anchored protein that can promote integrin-independent adhesion. Subsequent cleavage by metalloproteases, either constitutive or induced, can generate shed CX3CL1 entities that potentially have chemoattractive activity. To study the CX3C interface in tissues of live animals, we generated transgenic mice (CX3CL1cherry:CX3CR1gfp), which express red and green fluorescent reporter genes under the respective control of the CX3CL1 and CX3CR1 promoters. Furthermore, we performed a structure/function analysis to differentiate the in vivo functions of membrane-tethered versus shed CX3CL1 moieties by comparing their respective ability to correct established defects in macrophage function and leukocyte survival in CX3CL1-deficient mice. Specifically, expression of CX3CL1(105Δ), an obligatory soluble CX3CL1 isoform, reconstituted the formation of transepithelial dendrites by intestinal macrophages but did not rescue circulating Ly6Clo CX3CR1hi blood monocytes in CX3CR1gfp/gfp mice. Instead, monocyte survival required the full-length membrane-anchored CX3CL1, suggesting differential activities of tethered and shed CX3CL1 entities.
Kim KW, Vallon-Eberhard A, Zigmond E et al., Blood. 2011 Nov 24;118(22):e156-67. doi: 10.1182/blood-2011-04-348946. Epub 2011 Sep 27.
OBJECTIVE: Leukocyte infiltration of adipose is a critical determinant of obesity-related metabolic diseases. Fractalkine (CX3CL1) and its receptor (CX3CR1) comprise a chemokine system involved in leukocyte recruitment and adhesion in atherosclerosis, but its role in adipose inflammation and type 2 diabetes is unknown.
RESEARCH DESIGN AND METHODS:CX3CL1 mRNA and protein were quantified in subcutaneous adipose and blood during experimental human endotoxemia and in lean and obese human adipose. CX3CL1 cellular source was probed in human adipocytes, monocytes, and macrophages, and CX3CL1-blocking antibodies were used to assess its role in monocyte-adipocyte adhesion. The association of genetic variation in CX3CR1 with metabolic traits was determined in a community-based sample. Finally, plasma CX3CL1 levels were measured in a case-control study of type 2 diabetes.
RESULTS: Endotoxemia induced adipose CX3CL1 mRNA (32.7-fold, P < 1 × 10(-5)) and protein (43-fold, P = 0.006). Obese subjects had higher CX3CL1 levels in subcutaneous adipose compared with lean (0.420 ± 0.387 vs. 0.228 ± 0.187 ng/mL, P = 0.04). CX3CL1 was expressed and secreted by human adipocytes and stromal vascular cells. Inflammatory cytokine induction of CX3CL1 in human adipocytes (27.5-fold mRNA and threefold protein) was completely attenuated by pretreatment with a peroxisome proliferator-activated receptor-γ agonist. A putative functional nonsynonymous single nucleotide polymorphism (rs3732378) in CX3CR1 was associated with adipose and metabolic traits, and plasma CX3CL1 levels were increased in patients with type 2 diabetes vs. nondiabetics (0.506 ± 0.262 vs. 0.422 ± 0.210 ng/mL, P < 0.0001).
CONCLUSIONS: CX3CL1-CX3CR1 is a novel inflammatory adipose chemokine system that modulates monocyte adhesion to adipocytes and is associated with obesity, insulin resistance, and type 2 diabetes. These data provide support for CX3CL1 as a diagnostic and therapeutic target in cardiometabolic disease.
Shah R, Hinkle CC, Ferguson JF et al., Diabetes. 2011 May;60(5):1512-8. doi: 10.2337/db10-0956.
Soluble fractalkine plays a distinctive role in the inflammatory processes of the nervous system; however, the role of soluble fractalkine in Alzheimer's disease (AD) has not yet been investigated. In the present study, we evaluated the levels of plasma soluble fractalkine in patients with mild cognitive impairment (MCI), patients with AD and healthy controls. We also investigated the changes in the levels of plasma soluble fractalkine in patients with AD. A total of 102 patients with cognitive impairment, including 51 patients with MCI, 51 patients with AD, and 57 healthy control subjects, were enrolled in this study. The Mini-Mental Status Examination (MMSE) was used to evaluate the severity of cognitive impairment in patients with MCI and AD. The levels of plasma soluble fractalkine were measured using a specific enzyme-linked immunosorbent assay. There were significant group differences in the levels of plasma soluble fractalkine between the MCI, AD, and control groups. Post hoc analyses revealed significant differences between the MCI and control groups, the AD and control groups, and the MCI and AD groups. The level of plasma soluble fractalkine was significantly greater in the patients with mild to moderate AD than in the patients with severe AD. In addition, there was a positive correlation between MMSE score and plasma soluble fractalkine level in the patients with AD. This study provides preliminary evidence that soluble fractalkine is involved in the pathogenesis of AD.
Kim TS, Lim HK, Lee JY, et al., Neurosci Lett. 2008 May 9;436(2):196-200. doi: 10.1016/j.neulet.2008.03.019. Epub 2008 Mar 13.
Fractalkine, a novel CX3C chemokine, is unusual because of both its membrane-associated structure and its direct role in cell adhesion. We have solved the solution structure of the chemokine domain of fractalkine (residues 1-76) by heteronuclear NMR methods. The 20 lowest energy structures in the ensemble have an average backbone rmsd of 0.43 A, excluding the termini. In contrast to many other chemokines which form homodimers, fractalkine's chemokine module is monomeric. Comparison of the structure to CC and CXC chemokines reveals interesting differences which are likely to be relevant to receptor binding. These include a bulge formed by the CX3C motif, the relative orientation of the N-terminus and 30's loop (residues 30-38), and the conformation of the N-loop (residues 9-19). 15N backbone relaxation experiments indicate that these same regions of the protein are dynamic. We also titrated 15N-labeled protein with a peptide from the N-terminus of the receptor CX3CR1 and confirmed that this region of the receptor contacts the fractalkine chemokine domain. Interestingly, the binding site maps roughly to the regions of greatest flexibility and structural variability. Together, these data provide a first glimpse of how fractalkine interacts with its receptor and should help guide mutagenesis studies to further elucidate the molecular details of binding and signaling through CX3CR1.
Mizoue LS, Bazan JF, Johnson EC, Handel TM. Biochemistry. 1999 Feb 2;38(5):1402-14.
|
 |
|
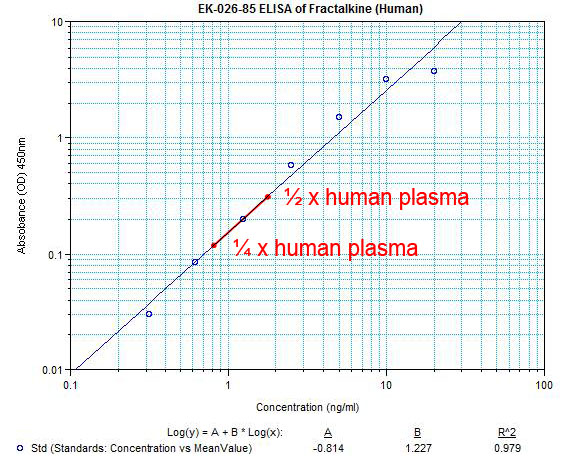
| Sensitivity: |
Minimum Detectable Concentration = 0.21 ng/ml |
Precision: |
Intra-assay variation: < 10 %
Inter-assay variation: < 15 % |
| Detection Range: |
0.3-20 ng/ml |
| |
|
|
|
%Fractalkine%
|
|
|


