|
|
|
Activation of GPR173 and GPR 101 by GnRH-(1-5) |
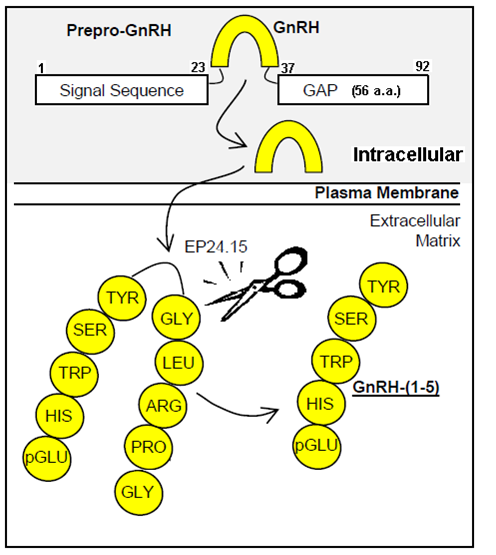
The prepro-GnRH is processed intracellularly to generate the mature GnRH peptide. In the extracellular matrix, GnRH is metabolized in a two step mechanism generating the metabolite GnRH-(1-5). Modified from Larco et al. (Front Endocrinol (Lausanne). 2013 Jul 8;4:83. )
The decapeptide GnRH is known for its central role in the regulation of the hypothalamo-pituitary-gonadal (HPG) axis. In addition, it is also known to have local effects within peripheral tissues. The zinc metalloendopeptidase, EC 3.4.24.15 (EP24.15), can cleave GnRH at the Tyr5-Gly6 bond to form the pentapeptide, GnRH-(1-5). The central and peripheral effect of GnRH-(1-5) is different from its parent peptide, GnRH. In the current study, we examined the effect of GnRH-(1-5) on EGFR phosphorylation and cellular migration. Using the Ishikawa cell line as a model of endometrial cancer, we demonstrate that GnRH-(1-5) stimulates EGF release, increases the phosphorylation of EGFR (p<0.05) at three tyrosine sites (992, 1045, 1068), and promotes cellular migration. In addition, we also demonstrate that these actions of GnRH-(1-5) are mediated by the orphan G-protein coupled receptor 101 (GPR101). Down-regulation of GPR101 expression blocked the GnRH-(1-5)-mediated release of EGF and the subsequent phosphorylation of EGFR and cellular migration. These results suggest that GPR101 is a critical requirement for GnRH-(1-5) transactivation of EGFR in Ishikawa cells.
Cho-Clark M, Larco DO, Semsarzadeh NN et al., Mol Endocrinol. 2013 Nov 21. [Epub ahead of print]
We have previously demonstrated that the cleavage product of the full-length GnRH, GnRH-(1-5), is biologically active, binds G protein-coupled receptor 173 (GPR173), and inhibits the migration of cells in the immortalized GnRH-secreting GN11 cell. In this study, we attempted to characterize the GnRH-(1-5) intracellular signaling mechanism. To determine whether the signaling pathway mediating GnRH-(1-5) regulation of migration involves a G protein-dependent mechanism, cells were treated with a generic G protein antagonist in the presence and absence of GnRH-(1-5), and a wound-healing assay was conducted to measure migration. G Protein antagonist 2 treatment abolished the GnRH-(1-5) inhibition of migration, indicating that the mechanism of GnRH-(1-5) is G protein coupled. To identify the potential Gα-subunit recruited by GnRH-(1-5) binding GPR173, we measured the second messengers cAMP and inositol triphosphate levels. GnRH-(1-5) treatment did not alter cAMP levels relative to cells treated with vehicle or forskolin, suggesting that GnRH-(1-5) does not couple to the Gαs or Gαi subunits. Similarly, inositol triphosphate levels remained unchanged with GnRH-(1-5) treatment, indicating a mechanism not mediated by the Gαq/11 subunit. Therefore, we also examined whether GnRH-(1-5) activating GPR173 deviated from the canonical G protein-coupled receptor signaling pathway by coupling to β-arrestin 1/2 to regulate migration. Our coimmunoprecipitation studies indicate that GnRH-(1-5) induces the rapid interaction between GPR173 and β-arrestin 2 in GN11 cells. Furthermore, we demonstrate that this association recruits phosphatase and tensin homolog to mediate the downstream action of GnRH-(1-5). These findings suggest that the GnRH-(1-5) mechanism deviates from the canonical G protein-coupled receptor pathway to regulate cell migration in immortalized GnRH neurons.
Larco DO, Semsarzadeh NN, Cho-Clark M et al., Endocrinology. 2013 Dec;154(12):4726-36. doi: 10.1210/en.2013-1286. Epub 2013 Oct 18.
The decapeptide GnRH is an important regulator of reproductive behavior and function. In the extracellular matrix, GnRH is metabolized by the endopeptidase EC3.4.24.15 (EP24.15) to generate the pentapeptide GnRH-(1-5). In addition to its expression in the adult hypothalamus, EP24.15 is expressed along the migratory path of GnRH-expressing neurons during development. Although we have previously demonstrated a role for EP24.15 in the generation of the biologically active pentapeptide GnRH-(1-5) in regulating GnRH expression and mediating sexual behavior during adulthood in rodents, the modulatory role of GnRH-(1-5) in the migration of GnRH neurons during development remains unknown. To address this information gap, we examined the effect of GnRH-(1-5) on the cellular migration of a premigratory GnRH-secreting neuronal cell line, the GN11 cell, using a wound-healing assay. Dose- and time-response studies demonstrated that GnRH-(1-5) significantly delayed wound closure. We then sought to identify the mechanism by which GnRH-(1-5) inhibits migration. Because the cognate GnRH receptor is a G protein-coupled receptor, we examined whether GnRH-(1-5) regulates migration by also activating a G protein-coupled receptor. Using a high-throughput β-arrestin recruitment assay, we identified an orphan G protein-coupled receptor (GPR173) that was specifically activated by GnRH-(1-5). Interestingly, small interfering RNA to GPR173 reversed the GnRH-(1-5)-mediated inhibition on migration of GN11 neurons. Furthermore, we also demonstrate that the GnRH-(1-5)-activated GPR173-dependent signal transduction pathway involves the activation of the signal transducer and activator of transcription 3 in GnRH migration. These findings indicate a potential regulatory role for GnRH-(1-5) in GnRH neuronal migration during development.
Larco DO, Cho-Clark M, Mani SK, Wu TJ. Endocrinology. 2013 Feb;154(2):783-95. doi: 10.1210/en.2012-1746. Epub 2013 Jan 15.
The gonadotropin-releasing hormone (GnRH) was originally isolated from the mammalian hypothalamus for its role as the primary regulator of reproductive function. Since its discovery, GnRH has also been shown to be located in non-hypothalamic tissues and is known to have diverse functions. Although the regulation of GnRH synthesis and release has been extensively studied, there is additional evidence to suggest that the processing of GnRH to the metabolite GnRH-(1-5) represents another layer of regulation. The focus of this review will be on the current evidence for the action of the pentapeptide metabolite GnRH-(1-5) in regulating cellular migration. We discuss the potential role of GnRH-(1-5) in regulating GnRH neuronal migration during development. Furthermore, we demonstrate these actions are mediated by the activation of a G protein-coupled receptor. Our findings suggest that GnRH-(1-5) may play a developmental function in addition to regulating developing cells.
Larco DO, Semsarzadeh NN, Cho-Clark M et al., Front Endocrinol (Lausanne). 2013 Jul 8;4:83. doi: 10.3389/fendo.2013.00083. eCollection 2013.
BACKGROUND:
Androgen deprivation therapy (ADT) is associated with increased cardiovascular morbidity.
OBJECTIVE:
To determine whether cardiovascular morbidity differs following initiation of gonadotropin-releasing hormone (GnRH) agonists compared with an antagonist.
DESIGN, SETTING, AND PARTICIPANTS:
Pooled data from six phase 3 prospective randomized trials that recruited 2328 men between 2005 and 2012 to compare the efficacy of GnRH agonists against an antagonist. Men recruited had pathologically confirmed prostate cancer, an Eastern Cooperative Oncology Group score <2, a minimum life expectancy of 12 mo, and were naïve to ADT. Men were excluded if they had a prolonged baseline QT/corrected QT interval, other risk factors for heart failure, hypokalemia or a family history of long QT syndrome, or had another cancer diagnosed within 5 yr.
INTERVENTION:
Men were randomized to receive a GnRH agonist or an antagonist for either 3-7 mo (n=642) or 12 mo (n=1686). Treatment groups were balanced for common baseline characteristics.
OUTCOME MEASUREMENTS AND STATISTICAL ANALYSIS:
Event analysis was based on death from any cause or cardiac events. Data documenting adverse experiences were classified based on the Medical Dictionary for Regulatory Activities. The following conditions defined a cardiac event: arterial embolic or thrombotic events, hemorrhagic or ischemic cerebrovascular conditions, myocardial infarction, and other ischemic heart disease. Kaplan-Meier curves and log-rank tests were used to compare time to a cardiovascular event or death.
RESULTS AND LIMITATIONS:
Among men with preexisting cardiovascular disease, the risk of cardiac events within 1 yr of initiating therapy was significantly lower among men treated with a GnRH antagonist compared with GnRH agonists (hazard ratio: 0.44; 95% confidence interval, 0.26-0.74; p=0.002). Since our analysis is post hoc, our findings should only be interpreted as hypothesis generating.
CONCLUSIONS:
GnRH antagonists appear to halve the number of cardiac events experienced by men with preexisting cardiovascular disease during the first year of ADT when compared to GnRH agonists.
Albertsen PC, Klotz L, Tombal B et al., Eur Urol. 2013 Nov 1. pii: S0302-2838(13)01108-1. doi: 10.1016/j.eururo.2013.10.032. [Epub ahead of print]
GHRH stimulates GH synthesis and release from the pituitary and exerts direct effects in extrapituitary tissues. We have previously shown that pretreatment with GHRH reduces cardiomyocyte apoptosis and improves heart function in isolated rat hearts subjected to ischemia/reperfusion (I/R). Here, we determined whether GHRH given at reperfusion reduces myocardial reperfusion injury and investigated the molecular mechanisms involved in GHRH effects. Isolated rat hearts subjected to I/R were treated at the onset of reperfusion with: 1) GHRH; 2) GHRH+GHRH antagonist JV-1-36; 3) GHRH+mitochondrial ATP-dependent potassium channel inhibitor 5-hydroxydecanoate; 4) GHRH+mitochondrial permeability transition pore opener atractyloside; 5) GHRH+ phosphoinositide 3-kinase/Akt inhibitor Wortmannin (WM); and 6) GHRH+signal transducer and activator of transcription-3 inhibitor tyrphostin-AG490 (AG490). GHRH reduced infarct size at the end of reperfusion and reverted contractility dysfunction in I/R hearts. These effects were inhibited by either JV-1-36, 5-hydroxydecanoate, atractylosid, WM, or AG490. Western blot analysis on left ventricles showed GHRH-induced phosphorylation of either the reperfusion injury salvage kinases (RISK), phosphoinositide 3-kinase/Akt, ERK1/2, and glycogen synthase kinase-3β or signal transducer and activator of transcription-3, as part of the survivor activating factor enhancement (SAFE) pathway. GHRH-induced activation of RISK and SAFE pathways was blocked by JV-1-36, WM, and AG490. Furthermore, GHRH increased the phosphorylation of endothelial nitric oxide synthase and AMP-activated protein kinase and preserved postischemic nicotinamide adenine dinucleotide (NAD(+)) levels. These results suggest that GHRH protects the heart from I/R injury through receptor-mediated mechanisms, leading to activation of RISK and SAFE pathways, which converge on mitochondria and possibly on AMP-activated protein kinase.
Penna C, Settanni F, Tullio F et al., Endocrinology. 2013 Apr;154(4):1624-35. doi: 10.1210/en.2012-2064. Epub 2013 Feb 15.
Idiopathic hypogonadotropic hypogonadism (IHH) is a condition characterized by failure to undergo puberty in the setting of low sex steroids and low gonadotropins. IHH is due to abnormal secretion or action of the master reproductive hormone gonadotropin-releasing hormone (GnRH). Several genes have been found to be mutated in patients with IHH, yet to date no mutations have been identified in the most obvious candidate gene, GNRH1 itself, which encodes the preprohormone that is ultimately processed to produce GnRH. We screened DNA from 310 patients with normosmic IHH (nIHH) and 192 healthy control subjects for sequence changes in GNRH1. In 1 patient with severe congenital nIHH (with micropenis, bilateral cryptorchidism, and absent puberty), a homozygous frameshift mutation that is predicted to disrupt the 3 C-terminal amino acids of the GnRH decapeptide and to produce a premature stop codon was identified. Heterozygous variants not seen in controls were identified in 4 patients with nIHH: 1 nonsynonymous missense mutation in the eighth amino acid of the GnRH decapeptide, 1 nonsense mutation that causes premature termination within the GnRH-associated peptide (GAP), which lies C-terminal to the GnRH decapeptide within the GnRH precursor, and 2 sequence variants that cause nonsynonymous amino-acid substitutions in the signal peptide and in GnRH-associated peptide. Our results establish mutations in GNRH1 as a genetic cause of nIHH.
Chan YM, de Guillebon A, Lang-Muritano M et al., Proc Natl Acad Sci U S A. 2009 Jul 14;106(28):11703-8. doi: 10.1073/pnas.0903449106. Epub 2009 Jun 30.
Gonadotrophin-releasing hormone (GnRH) was first isolated in the mammal and shown to be the primary regulator of the reproductive system through its initiation of pituitary gonadotrophin release. Subsequent to its discovery, this form of GnRH has been shown to be one of many structural variants found in the brain and peripheral tissues. Accordingly, the original form first discovered and cloned in the mammal is commonly referred to as GnRH-I. In addition to the complex regulation of GnRH-I synthesis, release and function, further evidence suggests that the processing of GnRH-I produces yet another layer of complexity in its activity. GnRH-I is processed by a zinc metalloendopeptidase EC 3.4.24.15 (EP24.15), which cleaves the hormone at the covalent bond between the fifth and sixth residue of the decapeptide (Tyr(5)-Gly(6)) to form GnRH-(1-5). It was previously thought that the cleavage of GnRH-I by EP24.15 represents the initiation of its degradation. Here, we review the evidence for the involvement of GnRH-(1-5), the metabolite of GnRH-I, in the regulation of GnRH-I synthesis, secretion and facilitation.
Wu TJ, Pagano E, Mani SK. J Neuroendocrinol. 2009 Mar;21(4):293-8. doi: 10.1111/j.1365-2826.2009.01854.x.

|
|
|
%GnRH%;%040-09%;%040-35%;%040-19%
|
|
|


