|
|
|
CCHamide |
New Peptides for Bombesin-receptor subtype 3 |
The insect neuropeptides CCHamide-1 and -2 are recently discovered peptides that probably occur in all arthropods. Here, we used immunocytochemistry, in situ hybridization, and quantitative PCR (qPCR), to localize the two peptides in the fruitfly Drosophila melanogaster. We found that CCHamide-1 and -2 were localized in endocrine cells of the midgut of larvae and adult flies. These endocrine cells had the appearance of sensory cells, projecting processes close to or into the gut lumen. In addition, CCHamide-2 was also localized in about forty neurons in the brain hemispheres and ventral nerve cord of larvae. Using qPCR we found high expression of the CCHamide-2 gene in the larval gut and very low expression of its receptor gene, while in the larval brain we found low expression of CCHamide-2 and very high expression of its receptor. These expression patterns suggest the following model: Endocrine CCHamide-2 cells in the gut sense the quality of food components in the gut lumen and transmit this information to the brain by releasing CCHamide-2 into the circulation; subsequently, after binding to its brain receptors, CCHamides-2 induces an altered feeding behavior in the animal and possibly other homeostatic adaptations.
Li S, Torre-Muruzabal T, Søgaard KC et al., PLoS One. 2013 Oct 2;8(10):e76131. doi: 10.1371/journal.pone.0076131.
Bombesin (Bn) is a 14-amino acid peptide isolated from the skin of the frog Bombina bombina. The mammalian homologs of this peptide include three forms of gastrin-releasing peptide (GRP): GRP-10, GRP-27, and GRP-29, and a 10-amino acid peptide referred to as neuromedin-B (NMB). These peptides evoke a number of responses, including hyperthermia, bradycardia, inhibition of gastric emptying and inhibition of food intake, by activating one of three G protein-coupled receptors: an NMB-R or BB(1), a GRP-R or BB(2) and an orphan Bn receptor subtype-3 (BRS-3) or BB(3). Bombesin, GRP, and NMB have a role in the short-term control of food intake. These peptides reduce meal size (MS) and they prolong the intermeal interval (IMI), the time between the first and second meals. Studies have shown that the vagus and the splanchnic nerves in the upper gastrointestinal tract, which communicate with the feeding areas of the hindbrain, are necessary for reduction of MS and prolongation of the IMI by Bn, GRP, and NMB. In addition, one-tenth of the intraperitoneal dose of Bn, GRP, and NMB given in either the left gastric artery, which supplies the stomach, or the cranial mesenteric artery, which supplies the intestine, or the femoral vein, also reduces MS and prolongs the IMI. Thus, a potential neurocrine or an endocrine mode of action for these peptides requires further investigation.
Sayegh AI. ProgMolBiolTransl Sci. 2013;114:343-70. doi: 10.1016/B978-0-12-386933-3.00010-8.
There are many orphan G protein-coupled receptors (GPCRs) for which ligands have not yet been identified. One such GPCR is the bombesin receptor subtype 3 (BRS-3). BRS-3 plays a role in the onset of diabetes and obesity. GPCRs in invertebrates are similar to those in vertebrates. Two Drosophila GPCRs (CG30106 and CG14593) belong to the BRS-3 phylogenetic subgroup. Here, we succeeded to biochemically purify the endogenous ligands of Drosophila CG30106 and CG14593 from whole Drosophila homogenates using functional assays with the reverse pharmacological technique, and identified their primary amino acid sequences. The purified ligands had been termed CCHamide-1 and CCHamide-2, although structurally identical to the peptides recently predicted from the genomic sequence searching. In addition, our biochemical characterization demonstrated two N-terminal extended forms of CCHamide-2. When administered to blowflies, CCHamide-2 increased their feeding motivation. Our results demonstrated these peptides actually present as the major components to activate these receptors in living Drosophila. Studies on the effects of CCHamides will facilitate the search for BRS-3 ligands.
Ida T et al., Front Endocrinol (Lausanne). 2012;3:177. doi: 10.3389/fendo.2012.00177. Epub 2012 Dec 31.
Bombesin receptor subtype-3 (BRS-3) regulates energy homeostasis, and BRS-3 agonism is being explored as a possible therapy for obesity. Here we study the role of BRS-3 in the regulation of glucose-stimulated insulin secretion (GSIS) and glucose homeostasis. We quantified BRS-3 mRNA in pancreatic islets from multiple species and examined the acute effects of Bag-1, a selective BRS-3 agonist, on GSIS in mouse, rat, and human islets, and on oral glucose tolerance in mice. BRS-3 is highly expressed in human, mouse, rhesus, and dog (but not rat) pancreatic islets and in rodent insulinoma cell lines (INS-1 832/3 and MIN6). Silencing BRS-3 with small interfering RNA or pharmacological blockade with a BRS-3 antagonist, Bantag-1, reduced GSIS in 832/3 cells. In contrast, the BRS-3 agonist (Bag-1) increased GSIS in 832/3 and MIN6 cells. The augmentation of GSIS by Bag-1 was completely blocked by U73122, a phospholipase C inhibitor. Bag-1 also enhanced GSIS in islets isolated from wild-type, but not Brs3 knockout mice. In vivo, Bag-1 reduced glucose levels during oral glucose tolerance test in a BRS-3-dependent manner. BRS-3 agonists also increased GSIS in human islets. These results identify a potential role for BRS-3 in islet physiology, with agonism directly promoting GSIS. Thus, in addition to its potential role in the treatment of obesity, BRS-3 may also regulate blood glucose levels and have a role in the treatment of diabetes mellitus.
Feng Y, et al., Endocrinology. 2011 Nov;152(11):4106-15. doi: 10.1210/en.2011-1440. Epub 2011 Aug 30.
Recently, a novel neuropeptide, CCHamide, was discovered in the silkworm Bombyx mori (L. Roller et al., Insect Biochem. Mol. Biol. 38
(2008) 1147-1157). We have now found that all insects with a sequenced genome have two genes, each coding for a different CCHamide,
CCHamide-1 and -2. We have also cloned and deorphanized two Drosophila G-protein-coupled receptors (GPCRs) coded for by genes CG14593 and
CG30106 that are selectively activated by Drosophila CCH-amide-1 (EC(50), 2×10(-9) M) and CCH-amide-2 (EC(50), 5×10(-9) M), respectively. Gene CG30106 (symbol synonym CG14484) has in a previous publication (E.C. Johnson et al., J. Biol. Chem. 278 (2003)
52172-52178) been wrongly assigned to code for an allatostatin-B receptor. This conclusion is based on our findings that the allatostatins-B do not activate the CG30106 receptor and on the recent findings from other research groups that the allatostatins-B activate an unrelated GPCR coded for by gene CG16752. Comparative genomics suggests that a duplication of the CCHamide neuropeptide signalling system occurred after the split of crustaceans and insects, about 410 million years ago, because only one CCHamide neuropeptide gene is found in the water flea Daphnia pulex (Crustacea) and the tick Ixodes scapularis (Chelicerata).
Hansen KK et al. Biochem Biophys Res Commun. 2011 Jan 7;404(1):184-9. doi: 10.1016/j.bbrc.2010.11.089.
The neuropeptides orexin A and orexin B (also known as hypocretin 1 and hypocretin 2), produced in lateral hypothalamic neurons, are critical regulators of feeding behavior, the reward system, and sleep/wake states. Orexin-producing neurons (orexin neurons) are regulated by various factors involved in regulation of energy homeostasis and sleep/wakefulness states. Bombesin receptor subtype 3 (BRS3) is an orphan receptor that might be implicated in energy homeostasis and is highly expressed in the hypothalamus. However, the neural pathway by which BRS3 regulates energy homeostasis is largely unknown. We examined whether BRS3 is involved in the regulation of orexin neurons. Using a calcium imaging method, we found that a selective BRS3 agonist [Ac-Phe-Trp-Ala-His-(tauBzl)-Nip-Gly-Arg-NH2] increased the intracellular calcium concentration of orexin neurons. However, intracellular recordings from slice preparations revealed that the BRS3 agonist hyperpolarized orexin neurons. The BRS3 agonist depolarized orexin neuron in the presence of tetrodotoxin. Moreover, in the presence of GABA receptor blockers, picrotoxin and CGP55845, the BRS3 agonist induced depolarization and increased firing frequency. Additionally, double-label in situ hybridization study revealed that Brs3 mRNA was expressed in almost all orexin neurons and many cells around these neurons. These findings suggest that the BRS3 agonist indirectly inhibited orexin neurons through GABAergic input and directly activated orexin neurons. Inhibition of activity of orexin neurons through BRS3 might be an important pathway for regulation of feeding and sleep/wake states. This pathway might serve as a novel target for the treatment of obesity.
Furutani N, et al, J MolNeurosci. 2010 Sep;42(1):106-11. doi: 10.1007/s12031-010-9382-5. Epub 2010 May 14.
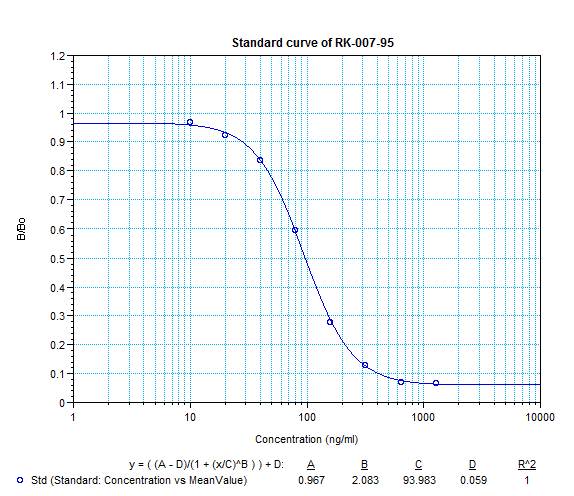 |
| Peptide |
% Cross-reactivity | CCHamide-2, Long Form
(Cat # 007-95) |
100 | CCHamide-2
(Cat # 007-93) |
0 | |
|
| |
|
|
%007-9%
|
|
|


