|
|
|
In1-Ghrelin |
A novel human ghrelin variant (in1-ghrelin) and ghrelin-o-acyltransferase
are overexpressed in breast cancer |
Encrinology annual conference 2015. Late break news, Sunday, March 8, 2015: 9:30 AM-11:00 AM
Ghrelin gene originates, through alternative splicing mechanisms and post-translational modifications, various peptides that regulate multiple, key pathophysiological processes, including tumoral processes. Our group has recently discovered a splicing variant of the ghrelin system, named In1-ghrelin, which results from the retention of intron 1. In1-ghrelin variant shares the initial portion of 13aa of its peptide sequence with native ghrelin, including the first 5-aa, which is the minimum sequence required for ghrelin acylation by ghrelin-O-acyl-transferase enzyme and for binding and activation of GHSR1a; whereas, the aa sequence of the C-terminal tail of In1-ghrelin is totally altered due to the retention of intron 1. In1-ghrelin variant has found to be overexpressed in breast cancer samples compared to control tissue, and its expression correlates with proliferation markers, suggesting the possible involvement of the In1-ghrelin in the development and/or progression of breast cancer. However, the role that In1-ghrelin plays in the regulation of key processes involved in the development and/or progression of breast cancer such as proliferation, migration and cell dedifferentiation [epithelial-mesenchymal transition (EMT) and mammosphere formation] is still unknown. The aim of this study was to determine the functional consequences associated with these tumor malignancy processes induced by the overexpression (using specific vector containing In1-ghrelin peptide) and/or silencing (using specific siRNAs of In1-ghrelin) of this variant by using the breast cancer cell line MDA-MB-231.
Overexpression of both In1-ghrelin and native ghrelin in MDA-MB-231 cells stimulated proliferation rate compared to control cells (72h after transfection; p =0.013 and 0.012, respectively). However, overexpression of In1-ghrelin, but not ghrelin, and also treatment with In1-ghrelin peptides increased the migratory ability of MDA-MB-231 cells (p=0.002) and also, induced an increase in p-ERK1/2 and a decreased in p-Akt. Migratory and proliferation ability is often associated to a change in cell differentiation status and, therefore, we determined the percentage of cells with mesenchymal-like phenotype and found that overexpression of In1-ghrelin, but not ghrelin, increased the number of cells with mesenchymal phenotype (p=0.013). We also studied the formation of mammospheres and found that only In1-ghrelin overexpression led to the formation of more, and bigger, mammospheres which could imply a greater proliferative capacity of cancer stem cells and, thus, a greater tumor growth and recurrence. Finally, we also found that In1-ghrelin silencing decreased proliferation and migration capacities of MDA-MB-231 cells. Altogether, our data demonstrate that In1-ghrelin variant overexpression increases malignancy features in breast cancer cells.
David Rincon Fernandez, Manuel D. Gahete, PhD, Virginia Ruiz, Raul M. Luque, PhD and Justo Pastor Castaño, PhD
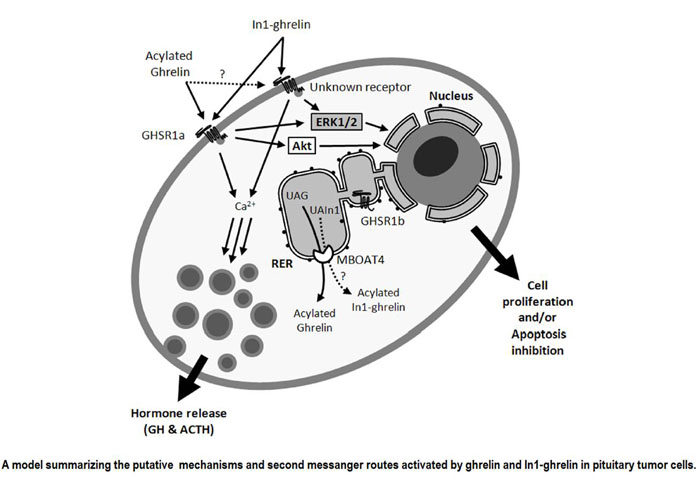
Figure from: Sci Rep. 2015 Mar 4;5:8714. doi: 10.1038/srep08714.
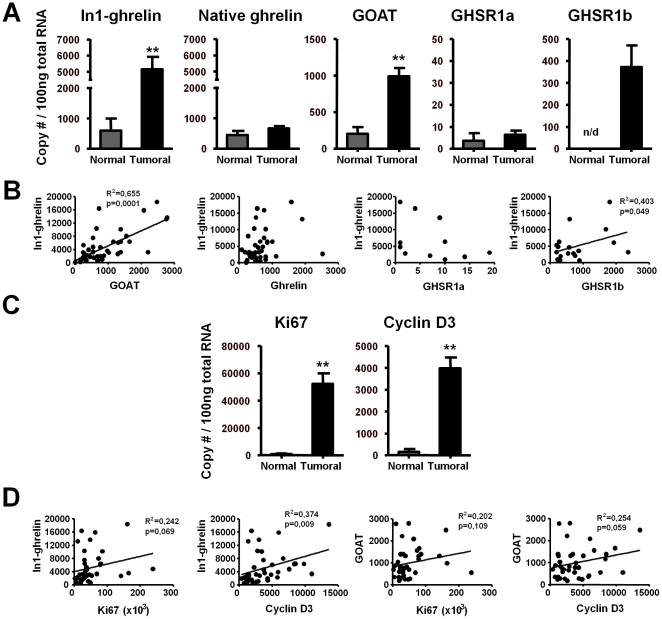
In1-ghrelin variant in human breast samples. Quantitative expression levels of the ghrelin-axis in normal (n = 4) and breast cancer samples (n = 40). (B) Correlations between In1-ghrelin and ghrelin, GOAT, GHSR1a/1b expression in breast cancer samples. (C) Quantitative expression levels of Ki67 and cyclin-D3 in normal and breast cancer samples. (D) Correlation values between In1-ghrelin and GOAT with Ki67 or cyclin-D3 expression in breast cancer samples. Values are shown as the mean ?S.E.M (** P less than 0.01; indicate differences from controls).
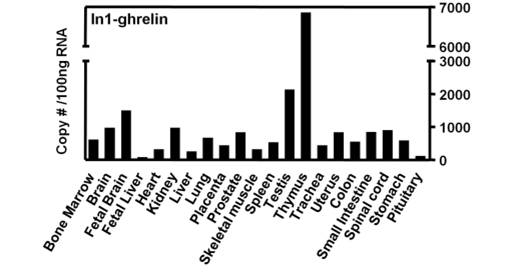
Tissue distribution of the human In1-ghrelin variant. Quantitative (qrtPCR) expression levels of In1-ghrelin using a commercial panel of total RNA from various human tissues obtained from Clontech, where each tissue sample is a pool of multiple individuals and represents a mean expression level in this particular tissue (only a single sample of each tissue is provided).
More information is provided in the published article.
Gahete MD, et al. Published in PLoS One. 2011;6(8):e23302. Epub 2011 Aug 4.
Pituitary adenomas comprise a heterogeneous subset of pathologies causing serious comorbidities, which would benefit from identification of novel, common molecular/cellular biomarkers and therapeutic targets. The ghrelin system has been linked to development of certain endocrine-related cancers. Systematic analysis of the presence and functional implications of some components of the ghrelin system, including native ghrelin, receptors and the recently discovered splicing variant In1-ghrelin, in human normal pituitaries (n=11) and pituitary adenomas (n=169) revealed that expression pattern of ghrelin system suffers a clear alteration in pituitary adenomasas compared with normal pituitary, where In1-ghrelin is markedly overexpressed. Interestingly, in cultured pituitary adenoma cells in1-ghrelin treatment (acylated peptides at 100 nM; 24-72h) increased GH and ACTH secretion, CA(2+) and ERK1/2 signaling and cell viabilty, whereas In1-ghrelin silencing (using a specific siRNA; 100nM) reduced cell viability.These results indicate that an alteration of the ghrelin system, specially its In1-ghrelin variant, could contribute to pathogenesis of different pituitary adenomas types, and suggest that this variant and its related ghrelin system could provide new tools to identify novel, more general diagnostic, prognostic and potential therapeutic targets in pituitary tumors.
Ibáñez-Costa A1, Gahete MD1, Rivero-Cortés E1 et al., Sci Rep. 2015 Mar 4;5:8714. doi: 10.1038/srep08714.
SCOPE: Dietary fat influences systemic inflammatory status, which determines the progression of age-associated diseases. Since somatostatin (SST), cortistatin (CORT), and ghrelin systems modulate inflammatory response, we aim to comprehensively characterize the presence and regulation of the components of these systems in the peripheral blood mononuclear cells (PMBCs), a subset of white blood cells placed at the crossroad between diet and inflammation, in response to diets with different fat composition, and during the postprandial phase in elderly subjects.
METHODS AND RESULTS: The applied nutrigenomic, inflammation-related PBMC-based approach revealed that the majority of components of SST/CORT and ghrelin systems are present in the human PBMCs. Particularly, CORT, SST/CORT receptors (sst2, sst3, sst5, and sst5TMD4), ghrelin, its acylating enzyme (GOAT), In1-ghrelin variant, and GHSR1b were detected in PBMCs. Their expression was altered in the long-term by diet composition, and in the short-term, during the postprandial phase. Of particular relevance is the postprandial elevation of CORT, sst2, and sst5 expression in PBMCs of subjects under n-3 PUFAs-enriched diet.
CONCLUSION: Our results suggest a potential relevant role of CORT/ssts and ghrelin systems in regulating PBMCs response to nutrient intake, which could help to explain the positive effects of n-3 PUFAs-enriched diets in reducing the inflammatory response.
Ghrelin is a 28-amino acid acylated hormone, highly expressed in the stomach, which binds to its cognate receptor (GHSR1a) to regulate a plethora of relevant biological processes, including food intake, energy balance, hormonal secretions, learning, inflammation, etc. However, ghrelin is, in fact, the most notorious component of a complex, intricate regulatory system comprised of a growing number of alternative peptides (e.g. obestatin, unacylated ghrelin, and In1-ghrelin, etc.), known (GHSRs) and, necessarily unknown receptors, as well as modifying enzymes (e.g. ghrelin-O-acyl-transferase), which interact among them as well as with other regulatory systems in order to tightly modulate key (patho)-physiological processes. This multiplicity of functions and versatility of the ghrelin system arise from a dual, genetic and functional, complexity. Importantly, a growing body of evidence suggests that dysregulation in some of the components of the ghrelin system can lead to or influence the development and/or progression of highly concerning pathologies such as endocrine-related tumors, inflammatory/cardiovascular diseases, and neurodegeneration, wherein these altered components could be used as diagnostic, prognostic, or therapeutic targets. In this context, the aim of this review is to integrate and comprehensively analyze the multiple components and functions of the ghrelin system described to date in order to define and understand its biological and (patho)-physiological significance.
Gahete MD1, Rincón-Fernández D, Villa-Osaba A et al., J Endocrinol. 2013 Dec 2;220(1):R1-24. doi: 10.1530/JOE-13-0391. Print 2014 Jan.
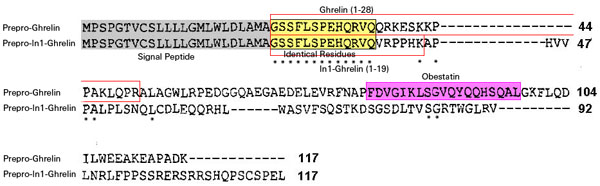
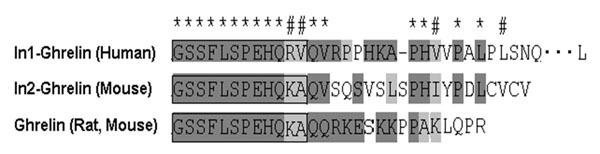
The human ghrelin gene, which encodes the ghrelin and obestatin peptides, contains 5 exons (Ex), with Ex1-Ex4 encoding a 117 amino-acid (aa) preproprotein that is known to be processed to yield a 28-aa (ghrelin) and/or a 23-aa (obestatin) mature peptides, which possess biological activities in multiple tissues. However, the ghrelin gene also encodes additional peptides through alternative splicing or post-translational modifications. Indeed, we previously identified a spliced mRNA ghrelin variant in mouse (In2-ghrelin-variant), which is regulated in a tissue-dependent manner by metabolic status and may thus be of biological relevance. Here, we have characterized a new human ghrelin variant that contains Ex0-1, intron (In) 1, and Ex2 and lacks Ex3-4. This human In1-ghrelin variant would encode a new prepropeptide that conserves the first 12aa of native-ghrelin (including the Ser3-potential octanoylation site) but has a different C-terminal tail. Expression of In1-variant was detected in 22 human tissues and its levels were positively correlated with those of ghrelin-O-acyltransferase (GOAT; p?=?0.0001) but not with native-ghrelin expression, suggesting that In1-ghrelin could be a primary substrate for GOAT in human tissues. Interestingly, levels of In1-ghrelin variant expression in breast cancer samples were 8-times higher than those of normal mammary tissue, and showed a strong correlation in breast tumors with GOAT (p=0.0001), ghrelin receptor-type 1b (GHSR1b; p=0.049) and cyclin-D3 (a cell-cycle inducer/proliferation marker; p=0.009), but not with native-ghrelin or GHSR1a expression. Interestingly, In1-ghrelin variant overexpression increased basal proliferation of MDA-MB-231 breast cancer cells. Taken together, our results provide evidence that In1-ghrelin is a novel element of the ghrelin family with a potential pathophysiological role in breast cancer.
Gahete et al., PLoS One. 2011;6(8):e23302. Epub 2011 Aug 4.
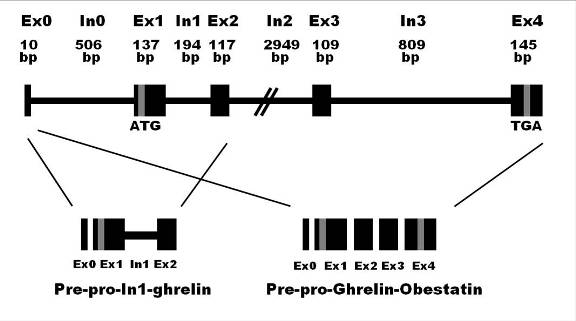
Classical organization of human ghrelin/obestatin gene consisting of 5 exons (Ex 0-4) and 4 introns (In 0-3).
The 28-aa mature ghrelin is encoded by Ex1-Ex2, while the 23 aa mature obestatin is encoded by Ex3 from the structure of prepro-ghrelin/obestatin.
The In1-ghrelin ghrelin variant shares the same start codon as native-ghrelin, located in the Ex1.
The translation of In1-ghrelin varient mRNA would encode a 117 aa protein from the prepro-In1-ghrelin mRNA precursors is proposed.
Gahete MD,et al. Published in PLoS One. 2011;6(8):e23302. Epub 2011 Aug 4.
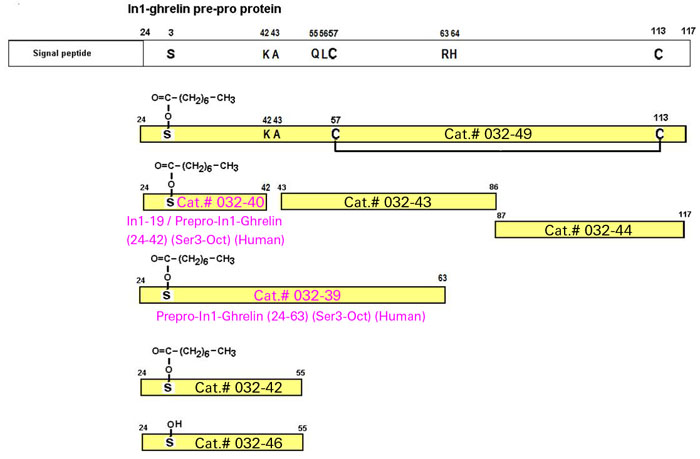
New Prepro-In1-Ghrelin RIA Kits Available!
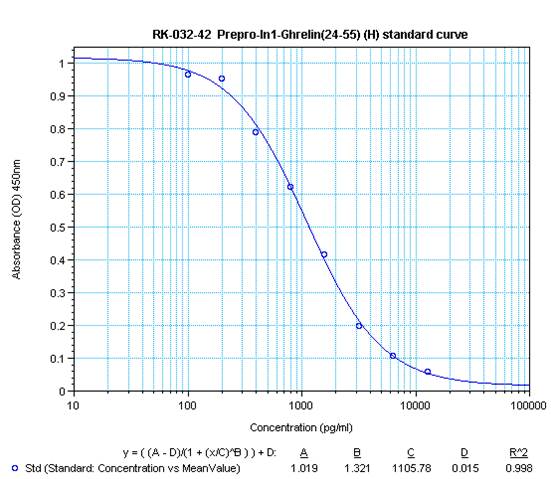
|
|
|
ghrelin_all;ghrelin_Goat;in2-ghrelin;obestatin
%prepro-in1%
|
|
|


