|
|
|
FGF-19 |
Postprandial insulin-independent activator of hepatic protein and glycogen synthesis |
Fibroblast growth factor (FGF) 19 is an enterokine synthesized and released when bile acids are taken up into the ileum. We show that FGF19 stimulates hepatic protein and glycogen synthesis but does not induce lipogenesis. The effects of FGF19 are independent of the activity of either insulin or the protein kinase Akt and, instead, are mediated through a mitogen-activated protein kinase signaling pathway that activates components of the protein translation machinery and stimulates glycogen synthase activity. Mice lacking FGF15 (the mouse FGF19 ortholog) fail to properly maintain blood concentrations of glucose and normal postprandial amounts of liver glycogen. FGF19 treatment restored the loss of glycogen in diabetic animals lacking insulin. Thus, FGF19 activates a physiologically important, insulin-independent endocrine pathway that regulates hepatic protein and glycogen metabolism.
Kir S, et al., Science. 2011 Mar 25;331(6024):1621-4. PMID: 21436455 [PubMed - indexed for MEDLINE]
Type 2 diabetes mellitus and its complications are, with cardiovascular diseases, leading threats to public health in the 21st century. In the United States, type 2 diabetes care accounts for a third of federal health insurance (Medicare) expenditures—nearly half of it to treat associated macrovascular problems (1). The cornerstone of type 2 diabetes is insulin resistance—a decreased sensitivity of tissues and organs, such as the liver, to the metabolic effects of the hormone insulin (the other major cause is failure of the pancreas to produce insulin). Yet, except for thiazolidinediones—whose checkered safety history, troublesome side effects, and regulatory setbacks stifled widespread adoption by clinicians—treatment options for insulin resistance have generally remained unchanged since the 1940s. Although insulin signaling pathways in cells have been largely deciphered (2), the key mediators of insulin signaling are poor drug targets because they either lack a suitable ligand-binding domain, or are shared with other cellular pathways that regulate cell growth and proliferation. This realization spawned research into “alternative pathways” that control insulin resistance. On page 1621 of this issue, Kir et al. (3) find that human fibroblast growth factor 19 (FGF19) can boost certain effects of insulin on the mammalian liver, raising interest and questions about possible therapies involving this molecule.
Kim-Muller JY, et. al., Cell Biology: Science. 2011 Mar 25;331(6024):1529-31. PMID: 21436429
FGF19 and FGF21 are distinctive members of the FGF family that function as endocrine hormones. Their potent effects on normalizing glucose, lipid, and energy homeostasis in disease models have made them an interesting focus of research for combating the growing epidemics of diabetes and obesity. Despite overlapping functions, FGF19 and FGF21 have many discrete effects, the most important being that FGF19 has both metabolic and proliferative effects, whereas FGF21 has only metabolic effects. Here we identify the structural determinants dictating differential receptor interactions that explain and distinguish these two physiological functions. We also have generated FGF19 variants that have lost the ability to induce hepatocyte proliferation but that still are effective in lowering plasma glucose levels and improving insulin sensitivity in mice. Our results add valuable insight into the structure-function relationship of FGF19/FGF21 and identify the structural basis underpinning the distinct proliferative feature of FGF19 compared with FGF21. In addition, these studies provide a road map for engineering FGF19 as a potential therapeutic candidate for treating diabetes and obesity.
Wu X, et al., Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14158-63. Epub 2010 Jul 26.
Fibroblast growth factors (Fgfs) are proteins with diverse functions in development, repair, and metabolism. The human Fgf gene family with 22 members can be classified into three groups, canonical, intracellular, and hormone-like Fgf genes. In contrast to canonical and intracellular Fgfs identified in invertebrates and vertebrates, hormone-like Fgfs, Fgf15/19, Fgf21, and Fgf23, are vertebrate-specific. The ancestral gene of hormone-like Fgfs was generated from the ancestral gene of canonical Fgfs by gene duplication early in vertebrate evolution. Later, Fgf15/19, Fgf21, and Fgf23 were generated from the ancestral gene by genome duplication events. Canonical Fgfs act as autocrine/paracrine factors in an Fgf receptor (Fgfr)-dependent manner. In contrast, hormone-like Fgfs act as endocrine factors in an Fgfr-dependent manner. Canonical Fgfs have a heparin-binding site necessary for the stable binding of Fgfrs and local signaling. In contrast, hormone-like Fgfs acquired endocrine functions by reducing their heparin-binding affinity during their evolution. Fgf15/19 and Fgf23 require βKlotho and αKlotho as cofactors, respectively. However, Fgf21 might physiologically require neither. Hormone-like Fgfs play roles in metabolism at postnatal stages, although they also play roles in development at embryonic stages. Fgf15/19 regulates bile acid metabolism in the liver. Fgf21 regulates lipid metabolism in the white adipose tissue. Fgf23 regulates serum phosphate and active vitamin D levels. Fgf23 signaling disorders caused by hereditary diseases or tumors result in metabolic disorders. In addition, serum Fgf19 or Fgf21 levels are significantly increased by metabolic disorders. Hormone-like Fgfs are newly emerging and quite unique in their evolution and function.
Itoh N., Cell Tissue Res. 2010 Oct; 342(1):1-11. Epub 2010 Aug 24. PMID: 20730630
Fibroblast growth factor 19 (FGF19) is a hormone-like protein that regulates carbohydrate, lipid and bile acid metabolism. At supra-physiological doses, FGF19 also increases hepatocyte proliferation and induces hepatocellular carcinogenesis in mice. Much of FGF19 activity is attributed to the activation of the liver enriched FGF Receptor 4 (FGFR4), although FGF19 can activate other FGFRs in vitro in the presence of the coreceptor ▀Klotho (KLB). In this report, we investigate the role of FGFR4 in mediating FGF19 activity by using Fgfr4 deficient mice as well as a variant of FGF19 protein (FGF19v) which is specifically impaired in activating FGFR4. Our results demonstrate that FGFR4 activation mediates the induction of hepatocyte proliferation and the suppression of bile acid biosynthesis by FGF19, but is not essential for FGF19 to improve glucose and lipid metabolism in high fat diet fed mice as well as in leptin-deficient ob/ob mice. Thus, FGF19 acts through multiple receptor pathways to elicit pleiotropic effects in regulating nutrient metabolism and cell proliferation.
Wu AL, et al., PLoS One. 2011 Mar 18;6(3):e17868. PMID: 21437243
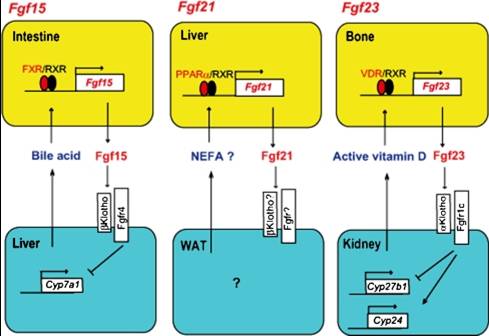
Action mechanisms of hormone-like Fgfs and regulatory mechanisms of their gene expression. (Fgf15) Intestinal Fgf15 expression is regulated by bile acid produced in the liver. The ligand-bound FXR forms a heterodimer with RXRs and induces the expression of Fgf15. The Fgf15 suppresses the expression of Cyp7a1 in the liver by activating the βKlotho-Fgfr4 complex. The regulatory process forms a negative feedback loop in the regulation of bile acid homeostasis by Fgf15. (Fgf21) Hepatic Fgf21 expression is induced by the activation of PPARα. NEFA binds to and activates PPARα. The ligand-bound PPARα forms a heterodimer with RXRs and induces the expression of Fgf21.
Itoh N. Cell Tissue Res. 2010 Oct;342(1):1-11
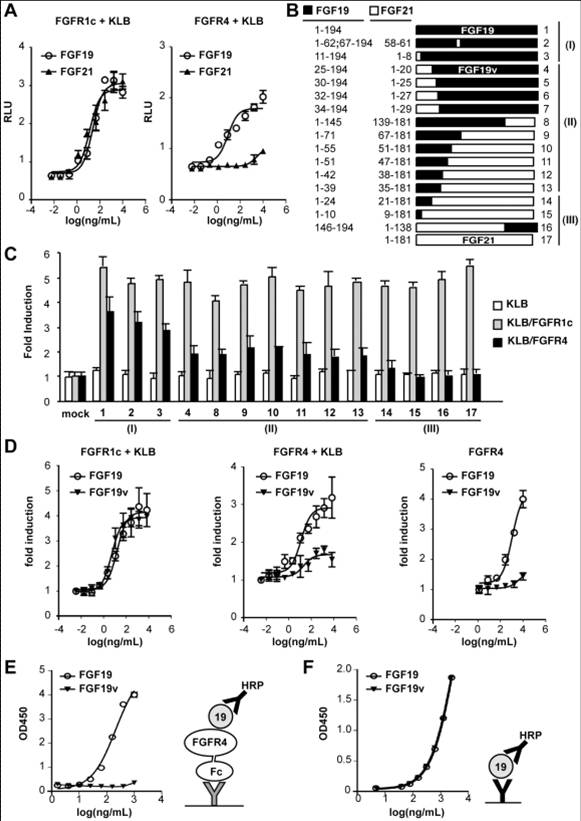
A) GAL-Elk1 luciferase assay in rat L6 cells. L6 cells were cotransfected with expression vectors for KLB and the indicated FGFR together with GAL-Elk1, SV40-renilla Luciferase, and Gal-responsive firefly luciferase reporter. Transfected cells were incubated with media containing increasing concentrations of FGF19 (○) or FGF21( ) for 6 hours before luciferase assays. Transcriptional activation was assessed by the relative firefly luciferase activity normalized by renilla luciferase activity and expressed as relative luciferase unit (RLU). (B) Drawings (to scale) of FGF19 (top), FGF21 (bottom), and various chimeric proteins with amino acid composition at left. Based on the results of repeated GAL-Elk1 assays such as shown in (C), each chimera was classified into class (I), (II) or (III) as indicated at right (see text). Chimeras which did not exhibit an equivalent FGFR1c activity to FGF21 or FGF19 when conditioned medium was used were not shown here. (C) Representative results of GAL-Elk-1 assay for chimeras shown in (B). L6 cells were cotransfected with expression vectors for KLB and/or FGFR as indicated at right. Each FGF construct was expressed in transiently transfected 293 cells and the conditioned medium was used in the assay. The results are shown as a fold induction over control media conditioned with mock transfected cells. (D) Similar to (A). Purified FGF19 (○) and FGF19v ( ), (the construct #4 in (B) and (C)), were tested for FGFR activation in the presence or absence of KLB coexpression as indicated. (E) Solid phase binding assay of FGF19 and FGF19v to FGFR4 fused to Fc fragment was tested as described in the method section. Schematic diagram for the experiments is shown at right. Bold Y indicates antibody against FGF19 (black) or Fc fragment (gray). HRP: horseradish peroxidase F) A control ELISA experiment to show that anti-FGF19 antibody used in (E) recognize FGF19 and FGF19v at indistinguishable affinity.
Wu A. L. et al, PLoS One. 2011 Mar 18;6(3):e17868
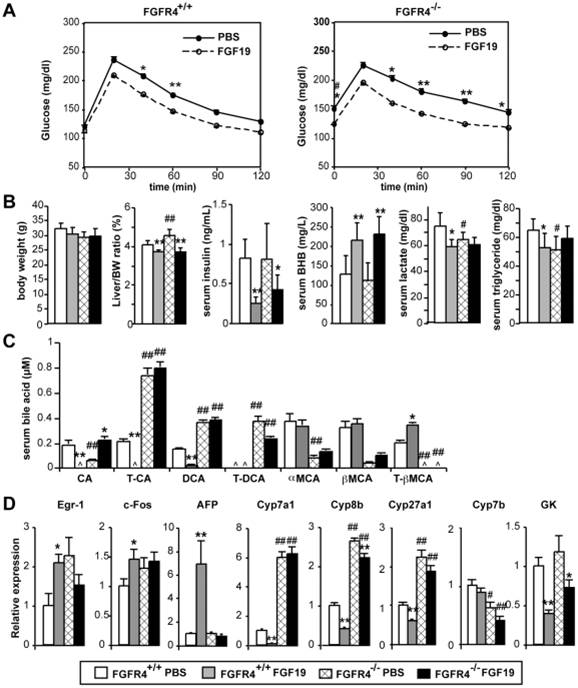
(A) WT and KO mice on high fat diet for 6 weeks were implanted with an osmotic pump to infuse FGF19 at 1 ng/hr (day 0). On day 6, overnight fasted mice were subjected to glucose tolerance test with i.p. injection of glucose at 1 g/kg. *p<0.05. **p<0.01. p value for area under the curve (AUC) was p<0.02 (WT) and p<0.005 (KO). N = 6~8. Note that fasting blood glucose levels were significantly higher in PBS-treated Fgfr4 KO mice compared with WT mice (#p<0.01). (B) Metabolic parameters at euthanasia on day 7. Mice were euthanized and serum prepared after 3 hr fast. N = 6~8. (C) Serum BA composition analysis. Only major BA species are shown. CA: cholic acid, DCA: deoxycholic acid, MCA: muricholic acid, T-:taurine-conjugated. ^: undetected. (D) Hepatic gene expression determined by real-time qPCR. N = 6~8. p values for (B), (C), and (D): <0.05, **<0.005 (PBS vs FGF19), #<0.05, ##<0.005 (WT vs Fgfr4KO).
Wu A. L. et al, PLoS One. 2011 Mar 18;6(3):e17868
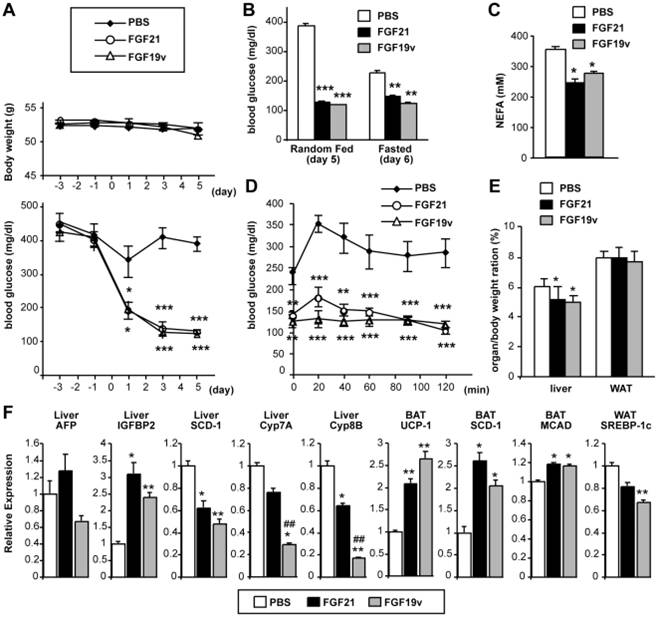
Wu A. L. et al, PLoS One. 2011 Mar 18;6(3):e17868
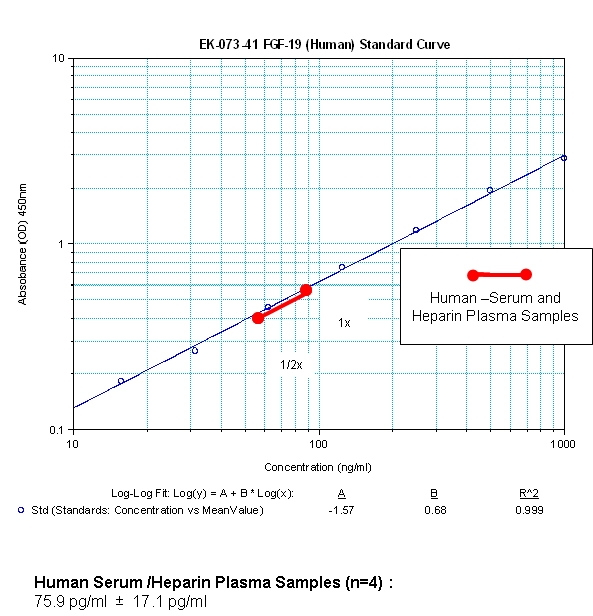
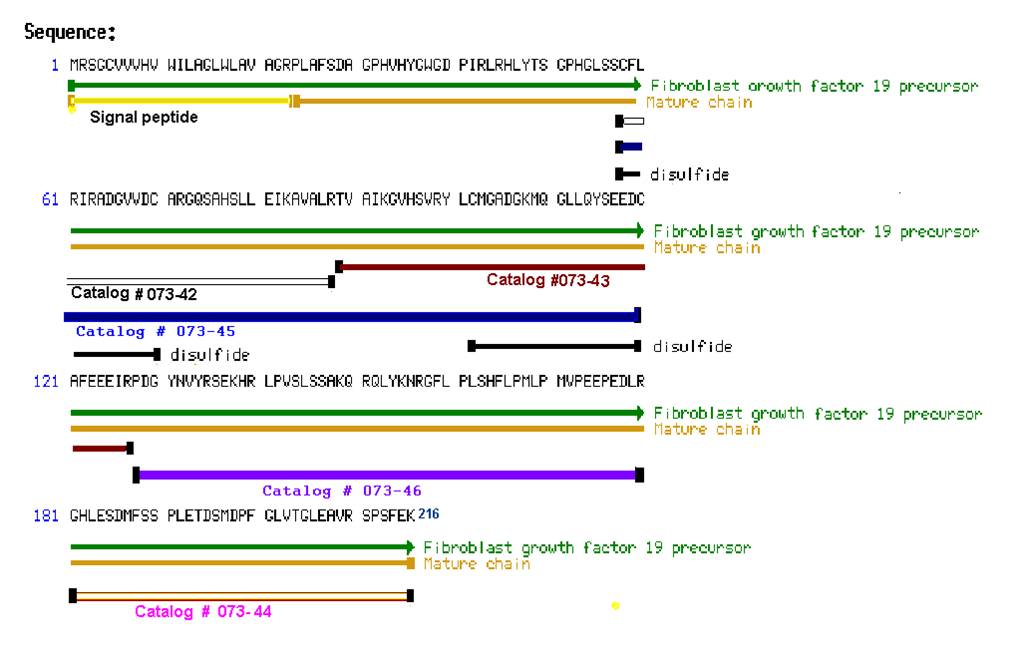
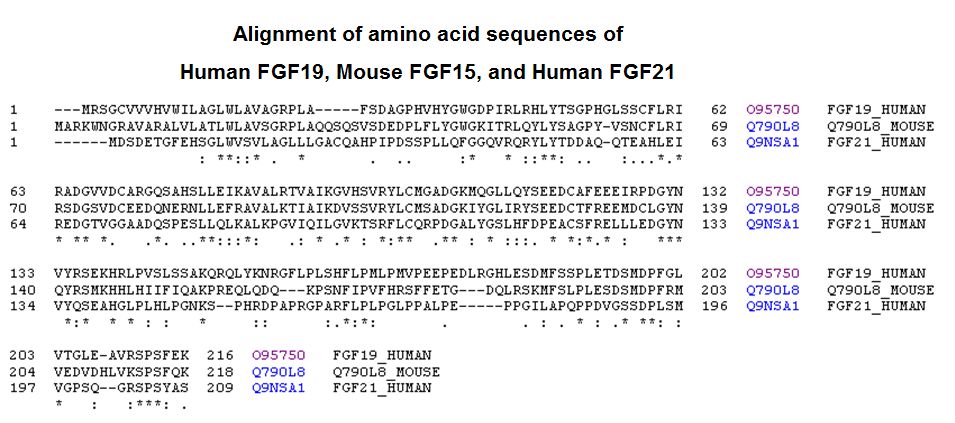
Tips: See More Research Abstracts, Antibody Stainings, Immunoassay Kits Curves and Sequences by clicking the tabs on the top.
|
|
|
FGF21;FGF23;FGF2
%fgf%
|
|
|


