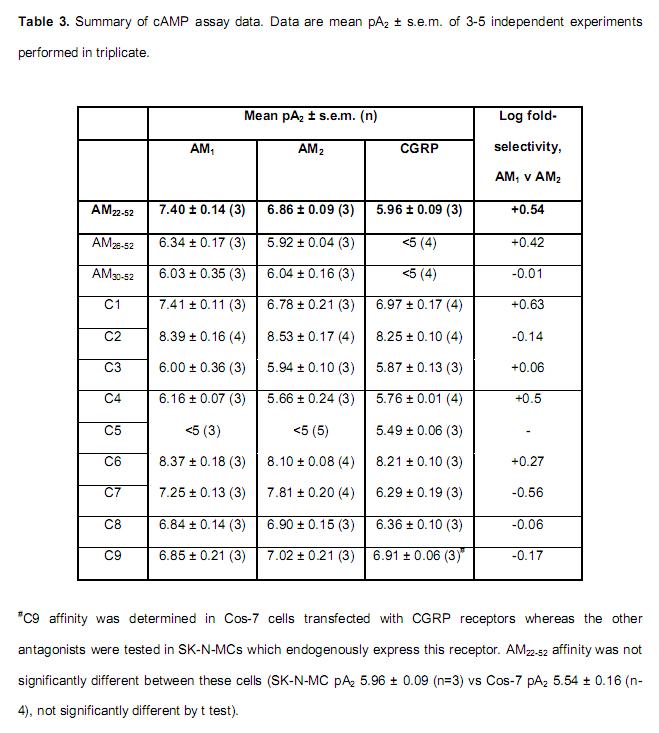
Human adrenomedullin (AM) is a 52 amino acid peptide belonging to the calcitonin peptide family, which also includes calcitonin gene-related peptide (alphaCGRP) and AM2. The two AM receptors, AM1 and AM2, are calcitonin receptor-like receptor (CL)/receptor activity-modifying protein (RAMP) (RAMP2 and RAMP3, respectively) heterodimers. CGRP receptors comprise CL/RAMP1. The only human AM receptor antagonist (AM22-52) is a truncated form of AM; it has low affinity and is only weakly selective for AM1 over AM2 receptors. To develop novel AM receptor antagonists we explored the importance of different regions of AM in interactions with AM1, AM2 and CGRP receptors. AM22-52 was the framework for generating further AM fragments (AM26-52 and AM30-52), novel AM/alphaCGRP (C1-C5, C9) and AM/AM2 chimeras (C6-C8). cAMP assays were used to screen the antagonists at all receptors to determine their affinity and selectivity. Circular dichroism spectroscopy was used to investigate the secondary structures of AM and its related peptides. The data indicate that the structures of AM, AM2 and alphaCGRP differ from one another. Our chimeric approach enabled the identification of two non-selective high-affinity antagonists of AM1, AM2 and CGRP receptors (C2, C6), one high-affinity antagonist of AM2 receptors (C7) and a weak antagonist selective for the CGRP receptor (C5). Using receptor mutagenesis we also determined that the C-terminal nine amino acids of AM appear to be responsible for its interaction with Glu 74 of RAMP3. We provide new information on the structure-activity relationship of AM, alphaCGRP and AM2 and how AM interacts with CGRP and AM2 receptors.
Robinson SD, et al. J Pharmacol Exp Ther. 2009 Jul 30. [Epub ahead of print]
Experimental studies have demonstrated that adrenomedullin (AM) has a positive inotropic action and exerts inhibitory effects against ventricular remodelling as an autocrine and paracrine factor. However, there is no clinical evidence for AM acting as a local regulator in the human heart. We measured the levels of various molecular forms of AM, i.e. an active form of mature AM (AM-m), an intermediate inactive form of glycine-extended AM (AM-Gly) and total AM (AM-T=AM-m+AM-Gly), in plasma and pericardial fluid using our newly developed immunoradiometric assay in consecutive 67 patients undergoing coronary artery bypass graft surgery. Pericardial fluid and plasma cAMP, atrial natriuretic peptide and brain natriuretic peptide levels were also measured. The relationships between pericardial fluid AM levels and ventricular functions and other hormone levels were analysed. The level of each molecular form of AM in pericardial fluid was closely correlated with that of the other molecular forms of AM in the fluid. However, levels were not correlated with those in plasma. AM-T levels were slightly higher in pericardial fluid than in plasma (+72%; P<0.05), whereas AM-m levels and AM-m/AM-T ratios were markedly higher in pericardial fluid than in plasma (AM-m, +994%; AM-m/AM-T ratio, +443%; both P<0.01). AM-m, AM-Gly and AM-T levels in pericardial fluid were correlated with indices of left ventricular function, and with atrial natriuretic peptide and brain natriuretic peptide levels. Interestingly, AM and cAMP levels were positively correlated in plasma, but negatively correlated in pericardial fluid. In addition, AM-m, AM-Gly and AM-T levels in pericardial fluid were higher in patients with acute coronary syndrome than in those with stable ischaemic heart disease (AM-m, +80%; AM-Gly, +96%; AM-T, +83%; all P<0.01). These results suggest that AM in pericardial fluid reflects cardiac synthesis, and that enhanced cardiac secretion of AM is associated with left ventricular dysfunction, ventricular overload and myocardial ischaemia. Considering that AM has positive inotropic, coronary vasodilatory and anti-remodelling actions, increased cardiac AM may play a compensatory role in the ischaemic and failing myocardium.
Nishikimi T, et al. Clin Sci (Lond). 2002 Jun;102(6):669-77