Gonad-stimulating substance (GSS) of starfish is the only known invertebrate peptide hormone responsible for final gamete maturation, rendering it functionally analogous to the vertebrate luteinizing hormone (LH). Here, we purified GSS of starfish, Asterina pectinifera, from radial nerves and determined its amino acid sequence. The purified GSS was a heterodimer composed of 2 different peptides, A and B chains, with disulfide cross-linkages. Based on its cysteine motif, starfish GSS was classified as a member of the insulin/insulin-like growth factor (IGF)/relaxin superfamily. The cDNA of GSS encodes a preprohormone sequence with a C peptide between the A and B chains. Phylogenetic analyses revealed that starfish GSS was a relaxin-like peptide. Chemically synthesized GSS induced not only oocyte maturation and ovulation in isolated ovarian fragments, but also unique spawning behavior, followed by release of gametes shortly after the injection. Importantly, the action of the synthetic GSS on oocyte maturation and ovulation was mediated through the production of cAMP by isolated ovarian follicle cells, thereby producing the maturation-inducing hormone of this species, 1-methyladenine. In situ hybridization showed the transcription of GSS to occur in the periphery of radial nerves at the side of tube feet. Together, the structure, sequence, and mode of signal transduction strongly suggest that GSS is closely related to the vertebrate relaxin.
Mita et al. Proc Natl Acad Sci U S A. 2009 Jun 9;106(23):9507-12.
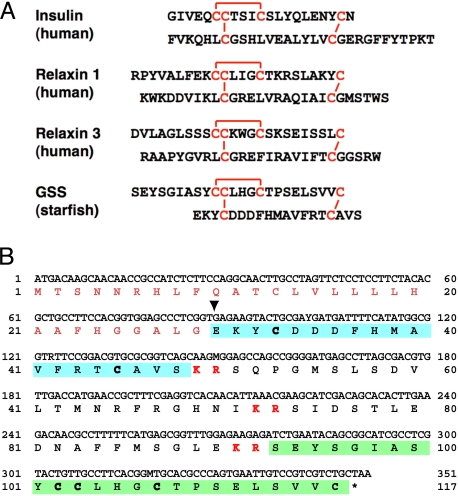
(A) Comparison of the heterodimeric structure of starfish GSS with those of various representative members of the insulin superfamily. The cysteine bridges are shown in red. (B) Coding DNA sequence and predicted amino acid sequences of GSS. Sequences of A and B chains are shown in green and blue boxes, respectively. Characters shown in red boldface indicate basic dipeptides that are the sites of proteolytic cleavage. Inverted triangle shows the deduced cleavage site of the signal peptide.
Mita et al. Proc Natl Acad Sci U S A. 2009 Jun 9;106(23):9507-12.
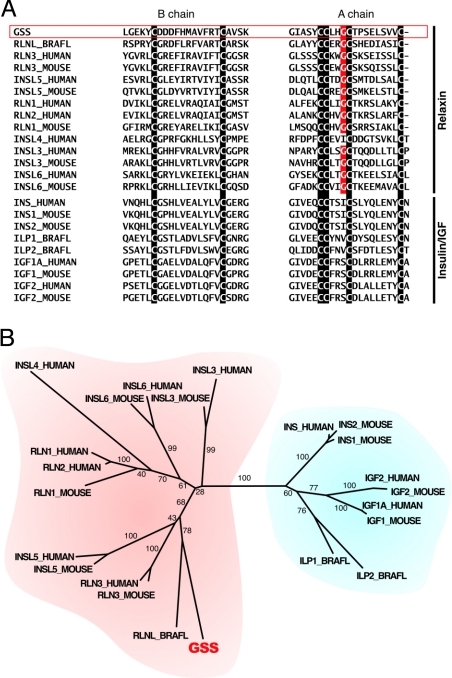
(A) Two parts of the whole peptide sequence alignment around the B and A chain regions. The cysteine residues that distinguish the insulin/IGF/relaxin superfamily peptides are indicated by the white characters on the black background. The glycine residues that are very common in the A peptides of the relaxin subfamily, but not in that of the insulin/IGF subfamily, are indicated by the white characters on the red background.
(B) The phylogenetic tree constructed from the alignment by using the neighbor-joining method. The number located beside each branch is the bootstrap score. The relaxin and the insulin/IGF subfamilies are indicated by red and blue backgrounds, respectively.
Mita et al. Proc Natl Acad Sci U S A. 2009 Jun 9;106(23):9507-12.


