58 Newly Identified Brain and Venom Peptides from Bees
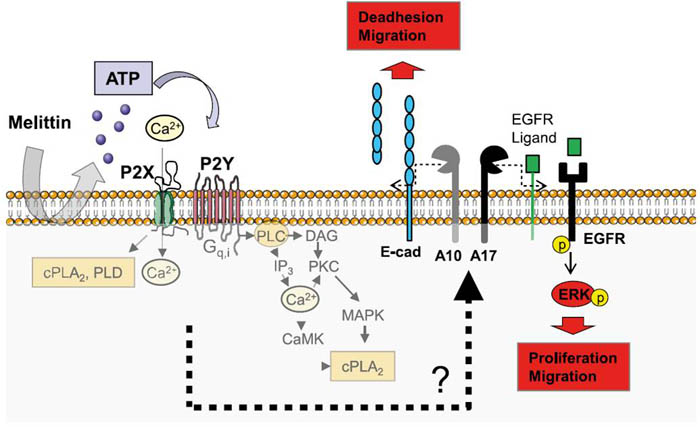
Metillin (cat. #070-50) induced ATO release lead to activation of P2X and P2Y receptors which trigger several signaling pathways.
Figure from: Melittin Modulates Keratinocyte Function through P2 Receptor-dependent ADAM Activation. Anselm Sommer. Et. al., J. Biol. Chem., Jul 2012; 287: 23678 - 23689.
 |
| 
|
|
| |
Peptide Sequence |
Catalog No. |
MW |
| 1 |
AYTYVSEY |
078-21 |
994.43 |
| 2 |
GRDYSFGL-Amide |
078-22 |
912.45 |
| 3 |
GRQPYSFGL-Amide |
078-23 |
1022.53 |
| 4 |
LPVYNFGI-Amide |
078-24 |
920.51 |
| 5 |
RQYSFGL-Amide |
078-25 |
868.46 |
| 6 |
GNNRPVYIPQPRPPHP |
078-03 |
1837.9 |
| 7 |
GNNRPVYIPQPRPPHPRL |
078-02 |
2107.14 |
| 8 |
PVYIPQPRPP |
078-08 |
1162.65 |
| 9 |
SFSENMINDHRQPAPTNNNY |
078-26 |
2348.02 |
| 10 |
DLSRFYGHFN |
078-27 |
1254.58 |
| 11 |
DLSRFYGHFNT |
078-28 |
1355.63 |
| 12 |
IDLSRFYGHF |
078-29 |
1253.62 |
| 13 |
IDLSRFYGHFN |
078-30 |
1367.66 |
| 14 |
IDLSRFYGHFNT |
078-31 |
1468.71 |
| 15 |
ITGQGNRIF |
078-32 |
1004.54 |
| 16 |
LRNQLDIGDL |
078-33 |
1155.62 |
| 17 |
LRNQLDIGDLQ |
078-34 |
1283.68 |
| 18 |
MVPVPVHHMADELLRNGPDTVI |
078-35 |
2439.24 |
| 19 |
VPVPVHHMADELL |
078-36 |
1455.75 |
| 20 |
QDVDHVFLRF-Amide |
039-09 |
1273.66 |
| 21 |
GIFLPGSVILRALSRQ-Amide |
078-37 |
1725.04 |
| 22 |
NIASLMRDYDQSRENRVPFP-Amide |
078-38 |
2406.19 |
| 23 |
NVASLARTYTLPQNA-Amide |
078-39 |
1616.86 |
| 24 |
NVGSVAREHGLPY-Amide |
078-40 |
1396.72 |
| 25 |
NVGTLARDFALPP-Amide |
078-41 |
1368.75 |
| 26 |
SVSSLAKNSAWPVSL |
078-42 |
1544.82 |
| 27 |
SVSSLARTGDLPVREQ |
078-43 |
1713.9 |
| 28 |
YVASLARTGLPIRGQ |
078-44 |
1715.78 |
| 29 |
NVPIYQEPRF |
078-45 |
1261.65 |
| 30 |
VPIYQEPRF |
078-46 |
1147.67 |
| 31 |
LTNYLATTGHGTNTGGPVLT |
078-47 |
1987.0 |
| 32 |
NIDEIDRTAFDNFF |
078-48 |
1715.78 |
| 33 |
NLDEIDRVGWSGFV |
078-49 |
1605.79 |
| 34 |
IYLPLFASRL-Amide |
078-50 |
1190.72 |
| 35 |
AYRKPPFNGSIF-Amide |
078-51 |
1394.75 |
| 36 |
NSELINSLLGLPKNMNNA-Amide |
078-52 |
1940.01 |
| 37 |
RKPPFNGSIF-Amide |
078-53 |
1160.65 |
| 38 |
SPSLRLRF-Amide |
078-54 |
973.59 |
| 39 |
ALMGFQGVR-Amide |
078-55 |
976.53 |
| 40 |
APMGFQGMRG |
078-56 |
1050.47 |
| 41 |
APMGFQGMR-Amide |
078-57 |
992.47 |
| 42 |
APMGFYGT |
078-58 |
842.36 |
| 43 |
APMGFYGTR-Amide |
078-59 |
997.48 |
| 44 |
ARMGFHGMR-Amide |
078-60 |
1060.52 |
| 45 |
ASFDDEYY |
078-61 |
1008.38 |
| 46 |
GVMDFQIGLQ |
078-62 |
1106.55 |
| 47 |
IILDALEELD |
078-63 |
1142.61 |
| 48 |
NSIINDVKNELFPEDIN |
078-64 |
1972.97 |
| 49 |
SLEEILDEI |
078-65 |
1059.54 |
| 50 |
SPFRYLGAR-Amide |
078-66 |
1064.59 |
| 51 |
GRNDLNFIRY-Amide |
078-67 |
1265.67 |
| 52 |
GIGAVLKVLTTGLPALISWIKRKRQQ-Amide |
070-50 |
2844.74 |
| 53 |
GNNRPIYIPQPRPPHP |
078-14 |
1853.12 |
| 54 |
PVYIPQPRPP |
078-08 |
1163.38 |
| 55 |
GNNRPVYIPQPRPPHPRL |
078-02 |
2108.44 |
| 56 |
GNNRPIYIPQPRPPHPRL |
078-01 |
2122.46 |
| 57 |
IKCNRKRHVIKPHICRKICGKN (Disulfide bridge Cys3 & Cys15, Cys5 & Cys19) |
004-04 |
2585.35 |
| 58 |
CNCKAPETALCARRCQQH (Disulfide Bridge: Cys3 - Cys15) |
063-12 |
2027.34 |
|
|
 |
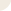
|
Infections by antibiotic-resistant bacteria are becoming a great risk for human health, leading to an urgent need for new efficient antibacterial therapies. The short, proline-rich antimicrobial peptides from insects gained a lot of interest as a potential antibacterial treatment, having a low toxicity profile and being mainly active against Gram-negative bacteria. To know whether these antimicrobial peptides can be used for the treatment of cerebral infections, the blood-brain barrier transport characteristics of these peptides were investigated. This study describes the results of the in vivo bloodbrain barrier experiments in mice, as well as the in vitro metabolic stability in mouse plasma and brain of apidaecin Api137, oncocin, drosocin and drosocin Pro5Hyp. The four investigated peptides showed a significant influx into the brain with a Kin ranging between 0.37 and 0.86 µL/g x min and brain distribution volumes of 19.6 to 25.8 µL/g. Only for drosocin, a significant efflux was determined, with a kout of 0.22 min(-1). After entering the brain, oncocin was for approximately 80% trapped in the endothelial cells, while the other peptides reached the brain parenchyma for about 70%. All peptides were stable in plasma and brain during the experiments, with estimated metabolic half-lives ranging between 47 min and 637 min. We conclude that the investigated short, proline-rich antimicrobial peptides show an influx into the brain, which make them a promising antibacterial treatment of cerebral infections.
Stalmans S, Wynendaele E, Bracke N et al., Protein Pept Lett. 2014 Apr;21(4):399-406.
Insect-derived antimicrobial peptides (AMPs) have diverse effects on antimicrobial properties and pharmacological activities such as anti-inflammation and anticancer properties. Naturally occurring genetic polymorphism have a direct and/or indirect influence on pharmacological effect of AMPs, therefore information on single nucleotide polymorphism (SNP) occurring in natural AMPs provides an important clue to therapeutic applications. Here we identified nucleotide polymorphisms in melittin gene of honey bee populations, which is one of the potent AMP in bee venoms. We found that the novel SNP of melittin gene exists in these two honey bee species, Apis mellifera and Apis cerana. Nine polymorphisms were identified within the coding region of the melittin gene, of which one polymorphism that resulted in serine (Ser) to asparagine (Asp) substitution that can potentially effect on biological activities of melittin peptide. Serine-substituted melittin (Mel-S) showed more cytotoxic effect than asparagine-substituted melittin (Mel-N) against E. coli. Also, Mel-N and Mel-S had different inhibitory effects on the production of inflammatory factors such as IL-6 and TNF-α in BV-2 cells. Moreover, Mel-S showed stronger cytotoxic activities than Mel-N peptide against two human ovarian cancer cell lines. Using carbon nanotube-based transistor, we here characterized that Mel-S interacted with small unilamellar liposomes more strongly than Mel-N. Taken together, our present study demonstrates that there exist different characteristics of the gene frequency and the biological activities of the melittin peptide in two honey bee species, Apis mellifera and A. cerana.
Park D, Jung JW, Lee MO et al., Peptides. 2014 Feb 8. pii: S0196-9781(14)00040-0. doi: 10.1016/j.peptides.2014.01.026. [Epub ahead of print]
Venoms are currently the focus of many drug discovery programs because they contain highly bioactive and selective components. Among them, apamin, a peptide found in bee venom, has received considerable attention because of its affinity for certain potassium channels and also because of its interesting structure and high stability to extreme pH and temperatures. Although apamin has long been claimed to cross the blood-brain barrier (BBB), only a few studies have been performed producing controversial results. In this article, it is shown that not only apamin is indeed able to penetrate the BBB in a cell-based model but also that an analog reported to be nontoxic passes through this barrier. Furthermore, the permeability values obtained, together with some evidence of an active transport mechanism and an amazing stability to serum proteases, make these peptides promising candidates for BBB shuttles.
Oller-Salvia B, Teixidó M, Giralt E, Biopolymers. 2013 Nov;100(6):675-86. doi: 10.1002/bip.22257.
Melittin (MEL) is a major peptide constituent of bee venom that has been proposed as one of the upcoming possibilities for anticancer therapy. Recent reports point to several mechanisms of MEL cytotoxicity in different types of cancer cells such as cell cycle alterations, effect on proliferation and/or growth inhibition, and induction of apoptotic and necrotic cell death trough several cancer cell death mechanisms, including the activation of caspases and matrix metalloproteinases. Although cytotoxic to a broad spectrum of tumour cells, the peptide is also toxic to normal cells. Therefore its therapeutic potential cannot be achieved without a proper delivery vehicle which could be overcome by MEL nanoparticles that possess the ability to safely deliver significant amount of MEL intravenously, and to target and kill tumours. This review paper summarizes the current knowledge and brings latest research findings on the anticancer potential of this lytic peptide with diverse functions.
Gajski G, Garaj-Vrhovac V, Environ Toxicol Pharmacol. 2013 Sep;36(2):697-705. doi: 10.1016/j.etap.2013.06.009. Epub 2013 Jun 28.
The honey bee genome predicts ≈100 peptides from 36 prohormones, but the functions of many of these peptides are unknown. We used differential isotope labeling combined with mass spectrometric analysis to quantify ≈50% of known bee brain peptides in the context of foraging, with 8 showing robust and dynamic regulation. Some showed differences in brain abundance as a function of experience; specifically, nectar and pollen collection led to quick changes in abundance. These changes were related to the act of food collection, not ingestion, because foragers bring food back to the hive for storage rather than eating it themselves. Other peptide differences in brain abundance were seen in bees that either flew to a nectar feeder or a pollen feeder, but did not yet collect any food. These differences likely reflect well-known predispositions of some bees to collect either nectar or pollen, but not both. Tachykinin, PBAN, and sNPF were among the peptides with the strongest changes in association with nectar and pollen foraging. These peptides are known to be involved in regulating food intake in solitary insects, suggesting an evolutionary connection between that behavior and social foraging. These results demonstrate that it is now possible to use quantitative peptidomics to help determine which brain peptides are bioactive and to elucidate their function in the regulation of behavior.
Published online before print January 28, 2009, doi: 10.1073/pnas.0813021106 PNAS February 17, 2009 vol. 106 no. 7 2383-2388
The Asiatic honeybee, Apis cerana Fabricius, is an important honeybee species in Asian countries. It is still found in the wild, but is also one of the few bee species that can be domesticated. It has acquired some genetic advantages and significantly different biological characteristics compared with other Apis species. However, it has been less studied, and over the past two decades, has become a threatened species in China. We designed primers for the sequences of the four antimicrobial peptide cDNA gene families (abaecin, defensin, apidaecin, and hymenoptaecin) of the Western honeybee, Apis mellifera L. and identified all the antimicrobial peptide cDNA genes in the Asiatic honeybee for the first time. All the sequences were amplified by reverse transcriptase-polymerase chain reaction (RT-PCR). In all, 29 different defensin cDNA genes coding 7 different defensin peptides, 11 different abaecin cDNA genes coding 2 different abaecin peptides, 13 different apidaecin cDNA genes coding 4 apidaecin peptides and 34 different hymenoptaecin cDNA genes coding 13 different hymenoptaecin peptides were cloned and identified from the Asiatic honeybee adult workers. Detailed comparison of these four antimicrobial peptide gene families with those of the Western honeybee revealed that there are many similarities in the quantity and amino acid components of peptides in the abaecin, defensin and apidaecin families, while many more hymenoptaecin peptides are found in the Asiatic honeybee than those in the Western honeybee (13 versus 1). The results indicated that the Asiatic honeybee adult generated more variable antimicrobial peptides, especially hymenoptaecin peptides than the Western honeybee when stimulated by pathogens or injury. This suggests that, compared to the Western honeybee that has a longer history of domestication, selection on the Asiatic honeybee has favored the generation of more variable antimicrobial peptides as protection against pathogens.
Xu P, Shi M, Chen XX, PLoS One. 2009;4(1):e4239. doi: 10.1371/journal.pone.0004239. Epub 2009 Jan 21.


