Chemotaxis of dendritic cells (DCs) and monocytes is a key step in the initiation of an adequate immune response. Formyl peptide receptor (FPR) and FPR-like receptor (FPRL)1, two G protein-coupled receptors belonging to the FPR family, play an essential role in host defense mechanisms against bacterial infection and in the regulation of inflammatory reactions. FPRL2, the third member of this structural family of chemoattractant receptors, is characterized by its specific expression on monocytes and DCs. Here, we present the isolation from a spleen extract and the functional characterization of F2L, a novel chemoattractant peptide acting specifically through FPRL2. F2L is an acetylated amino-terminal peptide derived from the cleavage of the human heme-binding protein, an intracellular tetrapyrolle-binding protein. The peptide binds and activates FPRL2 in the low nanomolar range, which triggers intracellular calcium release, inhibition of cAMP accumulation, and phosphorylation of extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases through the G(i) class of heterotrimeric G proteins. When tested on monocytes and monocyte-derived DCs, F2L promotes calcium mobilization and chemotaxis. Therefore, F2L appears as a new natural chemoattractant peptide for DCs and monocytes, and the first potent and specific agonist of FPRL2.
Migeotte I, et al. J Exp Med. 2005 Jan 3;201(1):83-93. Epub 2004 Dec 28
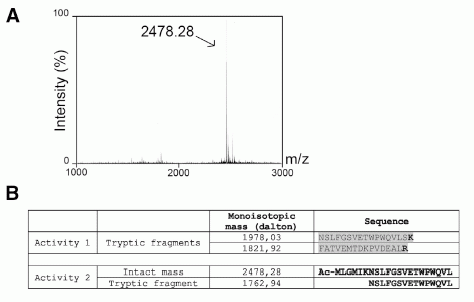

(A) Mass spectrometry analysis of the undigested fraction A2 resulting from step 4 using a Maldi Q-TOF mass spectrometer. (B) Sequences corresponding to the major peaks of the mass spectra of trypsin-digested A1 (step 5) and A2 (step 4) fractions or undigested A2. All microsequenced peptides were found to derive from the porcine HBP. The F2L peptide (A2 fraction) is amino terminally acetylated. Ac, acetyl. (C) Amino acid sequence alignment of human, mouse, and porcine HBP. The sequence corresponding to the F2L peptide (represented in bold) is identical in human and porcine HBP. The region containing tryptic peptides recovered from fraction A1 is boxed (human HBP, NM 015987; murine HBP, NM 013546). (D) The A1 fraction (step 5) was migrated onto SDS-PAGE together with 10, 50, and 100 ng aprotinin as standards. The gel was silver stained, and it was estimated that the major band (6 kD) contained around 300 ng of peptide. (E) Biological activity of the A1 fraction on CHO-K1 cells expressing human FPRL2 using the aequorin-based assay. (F) The A2 fraction (step 4) was migrated onto SDS-PAGE together with 10 and 50 ng aprotinin as standards. The gel was silver stained and the amount of F2L (3 kD) was estimated to 40 ng. (G) Biological activity of F2L (A2, step 4) on CHO-K1 cells expressing human FPRL2 using the aequorin-based assay.
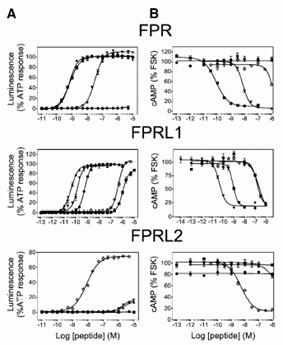

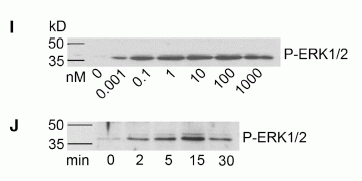
Pharmacology of the FPRs. (A) Concentration action curves of F2L ( ), FMLP ( ), WKYMVm ( ), WKYMVM ( ), and SHAAG ( ) peptides on CHO-K1 cells expressing FPR, FPRL1, or FPRL2 using the aequorin based assay. Results are expressed as percentage of the response elicited by 20 M ATP. (B) Concentration action curves of the same peptides on CHO-K1 cells expressing the three receptors using a cAMP accumulation assay. Results are expressed as percentage of the cAMP level obtained in the presence of 10 M forskolin but in the absence of agonists. FSK, forskolin. (C) Saturation binding assay (specific binding) on FPRL2-expressing CHO-K1 cells using F2L bearing a carboxy-terminal [125I]Tyr as tracer. (D) Competition binding assay on FPRL2-expressing CHO-K1 cells using F2L-[125I]Tyr as tracer and F2L as competitor. (E and F) Competition binding assay on (E) FPRL1- and (F) FPR-expressing CHO-K1 cells using [125I]-WKYMVm as tracer and WKYMVm ( ) or F2L ( ) as competitors. (G) Stimulation by F2L of FPRL2-expressing CHO-K1 cells cultured in the absence or presence of 100 ng/ml pertussis toxin using the aequorin-based assay. (H) Concentration action curves of acetylated ( ), nonacetylated ( ), and [7–21]F2L () peptides on FPRL2-expressing CHO-K1 cells using the aequorin assay. (I) Immunodetection of phosphorylated ERK1/2 in FPRL2-expressing CHO-K1 cells after stimulation by F2L for 10 min. (J) Kinetics of ERK1/2 activation after stimulation by 100 nM F2L. Each experiment displayed in A–I was repeated at least three times.

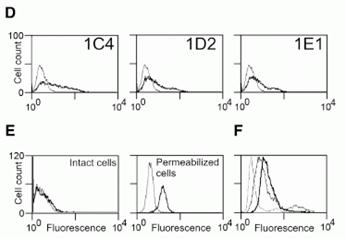
Expression profile of human FPRL2. (A) Transcripts encoding human FPRL2 were amplified by RT-PCR in a set of human leukocyte populations. act., activated; Mononucl., mononuclear cells; iDC, immature DCs; mDC, mature DCs. (B) Distribution of FPRL2 in a set of human tissues by using quantitative RT-PCR (Taqman). The data were normalized for the expression of GAPDH used as control. (C) Anti-FPRL2 monoclonal antibodies were characterized by FACS on CHO-K1 cells expressing FPR, FPRL1, and FPRL2. Bold solid line, 1C4; dotted line, 1D2; dashed line, 1E1; thin solid line, control labeling (IgG2a). The profiles of 1D2 and 1E1 are superimposed and cannot therefore be distinguished. (D) The expression of FPRL2 was analyzed by FACS on immature DCs using the three monoclonal antibodies. Bold solid line, anti-FPRL2 antibodies; thin solid line, control labeling (IgG2a). (E) Expression of FPRL2 on intact and permeabilized DCs using 1C4. Bold solid line, 1C4; thin solid line, control labeling (IgG2a). (F) Expression of FPRL2 on immature (bold solid line) and mature (thin solid line) DCs using
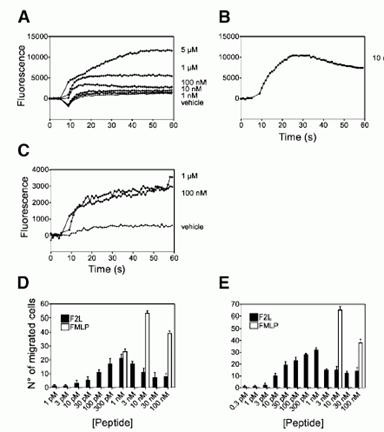
Biological activity of F2L on primary immune cells. (A and B) Recording of Ca2 flux in monocyte-derived DCs in response to various concentrations of F2L (A) and 10 nM FMLP (B). (C) Recording of Ca2 flux in monocytes in response to 100 nM and 1 M F2L. (D and E) Chemotaxis of monocyte-derived human immature DCs (D) and PBMCs (E) in response to F2L. The displayed responses are representative of four donors out of five tested.


