Genomic structure, promoter region, amino acid sequence and exon-specific primer combinations of the human omentin gene are presented. Omentin mRNA expression differs between omental adipose tissue probes from patients with chronic inflammatory bowel diseases such as Crohn's disease. Sequence comparisons revealed a 100% identity of omentin with human intelectin. Based on this, omentin might be a new adipocytokine playing a role in the defense against intestinal bacterial translocation in the context of Crohn's disease.
Schaffler A, et al. Biochim Biophys Acta. 2005 Dec 19
Various adipocyte-secreted factors have been described which profoundly affect insulin sensitivity and might potentially link obesity, insulin resistance and cardiovascular disease. Among those, adiponectin, visfatin and omentin appear as insulin-sensitising adipocytokines, whereas TNF-alpha, IL-6 and resistin induce insulin resistance. Moreover, leptin is a fat-derived key regulator of appetite and energy expenditure. Due to their profound effect on whole-body glucose and energy metabolism, adipocytokines have attracted interest as potential new therapeutics for diabetes mellitus and obesity. The current knowledge on function, regulation and therapeutic potential of various adipocytokines, as well as their clinical implications, are discussed in this review.
Kralisch S, et al. Expert Opin Pharmacother. 2005 Jun;6(6):863-72.
Malignant pleural mesothelioma (MPM) is a fatal neoplasm with no acceptable curative approaches. We used serial analysis of gene expression (SAGE) to compare the gene expression pattern of a surgically resected MPM to the autologous normal mesothelium. Intelectin gene overexpression (>139-fold) was found in the tumor. Online SAGE datasets revealed intelectin to be consistently present in mesothelioma(s), ovarian cancer, and colon cancer. Intelectin mRNA expression was found by RT-PCR in 4 of 5 resected MPM tumors, and Intelectin protein expression was confirmed by immunohistochemistry in 28 of 53 MPM tumors, and in 4 of 4 mesothelioma cell lines studied by Western blot. A marked induction in intelectin gene expression was observed among human primary mesothelial cells as a consequence of crocidolite asbestos exposure and simian virus 40 infection. Intelectin overexpression in mesothelioma could have potential screening, and therapeutic implications.
Wali A, et al. Lung Cancer. 2005 Apr;48(1):19-29. Epub 2004 Dec 18
Galactofuranosyl residues are present in various microorganisms but not in mammals. In this study, we identified a human lectin binding to galactofuranosyl residues and named this protein human intelectin (hIntL). The mature hIntL was a secretory glycoprotein consisting of 295 amino acids and N-linked oligosaccharides, and its basic structural unit was a 120-kDa homotrimer in which 40-kDa polypeptides were bridged by disulfide bonds. The hIntL gene was split into 8 exons on chromosome 1q21.3, and hIntL mRNA was expressed in the heart, small intestine, colon, and thymus. hIntL showed high levels of homology with mouse intelectin, Xenopus laevis cortical granule lectin/oocyte lectin, lamprey serum lectin, and ascidian galactose-specific lectin. These homologues commonly contained no carbohydrate recognition domain, which is a characteristic of C-type lectins, although some of them have been reported as Ca(2+)-dependent lectins. Recombinant hIntL revealed affinities to d-pentoses and a d-galactofuranosyl residue in the presence of Ca(2+), and recognized the bacterial arabinogalactan of Nocardia containing d-galactofuranosyl residues. These results suggested that hIntL is a new type lectin recognizing galactofuranose, and that hIntL plays a role in the recognition of bacteria-specific components in the host.
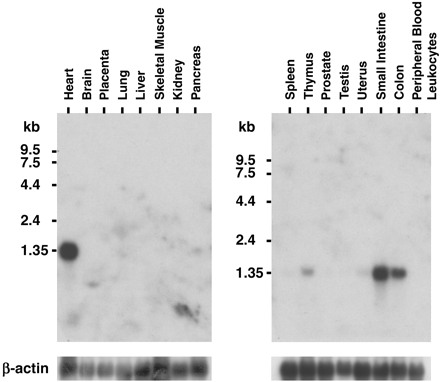
Northern blotting of hIntL mRNA. MTN blot membranes (CLONTECH) were hybridized with the cDNA fragment corresponding to the open reading frame of hIntL as described under "Experimental Procedures." RNA size markers are indicated in kilobase pairs (kb).
Tsuji S., et al. J. Biol. Chem., Vol. 276, Issue 26, 23456-23463, June 29, 2001
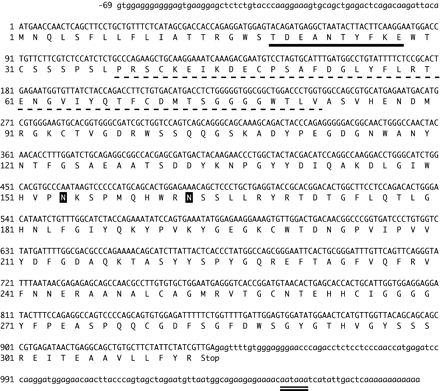
The nucleotide sequence (upper) and the deduced amino acid sequence (lower) are numbered (GenBankTM accession no. AB036706). The N-terminal amino acid sequence of hIntL purified from the placenta is indicated by the solid underline. The broken underline indicates a sequence similar to part of the fibrinogen domain. The amino acids in black boxes are potential N-glycosylation sites. The double underline indicates the position of the polyadenylation signal.

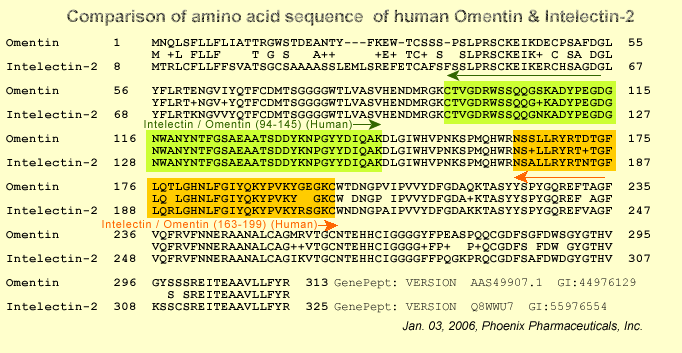
Comparison of amino acid sequences of hIntL and its homologues. Amino acid sequences of hIntL, Mus musculus mouse intelectin (mIntL), X. laevis cortical granule lectin (xCGL), L. japonica lamprey serum lectin (lSL), and H. roretzi ascidian galactose-specific lectin (aGSL) are shown. Sequence information was obtained from the GenBankTM data base or the literature as follows: M. musculus mouse intelectin , AB016496; X. laevis cortical granule lectin, X82626; L. japonica lamprey serum lectin, AB055981; H. roretzi ascidian galactose-specific lectin, see Ref. 27. The highly homologous amino acids are shown in shaded characters, and identical amino acids in all proteins are marked in the consensus row.
Tsuji S, et al. J Biol Chem. 2001 Jun 29;276(26):23456-63


