|
|
|
DX 600 & WFML |
A
novel ACE2-specific peptide inhibitor & Rat Renin Inhibitor |
Angiotensin-converting enzyme 2 (ACE2), a recently
identified human homolog of ACE, is a novel
metallocarboxypeptidase with specificity, tissue distribution,
and function distinct from those of ACE. ACE2 may play a
unique role in the renin-angiotensin system and mediate
cardiovascular and renal function. Here we report the
discovery of ACE2 peptide inhibitors through selection of
constrained peptide libraries displayed on phage. Six
constrained peptide libraries were constructed and selected
against FLAG-tagged ACE2 target. ACE2 peptide binders were
identified and classified into five groups, based on their
effects on ACE2 activity. Peptides from the first three
classes exhibited none, weak, or moderate inhibition on ACE2.
Peptides from the fourth class exhibited strong inhibition,
with equilibrium inhibition constants (K(i) values) from 0.38
to 1.7 microm. Peptides from the fifth class exhibited very
strong inhibition, with K(i) values < 0.14 microm. The most
potent inhibitor, DX600, had a K(i) of 2.8 nm. Steady-state
enzyme kinetic analysis showed that these potent ACE2
inhibitors exhibited a mixed competitive and non-competitive
type of inhibition. They were not hydrolyzed by ACE2.
Furthermore, they did not inhibit ACE activity, and thus were
specific to ACE2. Finally, they also inhibited ACE2 activity
toward its natural substrate angiotensin I, suggesting that
they would be functional in vivo. As novel ACE2-specific
peptide inhibitors, they should be useful in elucidation of
ACE2 in vivo function, thus contributing to our better
understanding of the biology of cardiovascular regulation. Our
results also demonstrate that library selection by phage
display technology can be a rapid and efficient way to
discover potent and specific protease inhibitors.
Huang L, et al. J Biol Chem. 2003
May 2;278(18):15532-40.
The mammalian brain
harbors a renin-angiotensin system (RAS), which is independent
from the peripheral RAS. Angiotensin II is a well-studied
member of the RAS and exerts most of the known
angiotensin-mediated effects on fluid and electrolyte
homeostasis, autonomic activity, neuroendocrine regulation,
and behavior. This review summarizes a mass of compelling new
evidence for the biological role of an active (3-8) fragment
of angiotensin II, named angiotensin IV. Angiotensin IV binds
to a widely distributed binding site in the brain, but which
is different from the known angiotensin II receptors AT1 and
AT2. Angiotensin IV has been implicated in a number of
physiological actions, including the regulation of blood flow,
the modulation of exploratory behavior, and processes
attributed to learning and memory. Furthermore, angiotensin IV
may also be involved in neuronal development. Collectively,
the available evidence suggests that angiotensin IV is a
potent neuropeptide, involved in a broad range of brain
functions.
Von Bohlen Und
Halbach O. Cell Tissue Res 2003
Jan;311(1):1-9
Cardiovascular diseases are predicted to
be the most common cause of death worldwide by 2020. Here we
show that angiotensin-converting enzyme 2 (ace2) maps to a
defined quantitative trait locus (QTL) on the X chromosome in
three different rat models of hypertension. In all
hypertensive rat strains, ACE2 messenger RNA and protein
expression were markedly reduced, suggesting that ace2 is a
candidate gene for this QTL. Targeted disruption of ACE2 in
mice results in a severe cardiac contractility defect,
increased angiotensin II levels, and up regulation of
hypoxia-induced genes in the heart. Genetic ablation of ACE on
an ACE2 mutant background completely rescues the cardiac
phenotype. But disruption of ACER, a Drosophila ACE2
homologue, results in a severe defect of heart morphogenesis.
These genetic data for ACE2 show that it is an essential
regulator of heart function in vivo.
Crackower MA, et al. Nature. 2002 Jun
20;417(6891):822-8.
Although angiotensin IV (Ang IV) was
thought initially to be an inactive product of Ang II
degradation, it was subsequently shown that the hexapeptide
markedly enhances learning and memory in normal rodents and
reverses the memory deficits seen in animal models of amnesia.
These central nervous system effects of Ang IV are mediated by
binding to a specific site, known as the AT(4) receptor, which
is found in appreciable levels throughout the brain and is
concentrated particularly in regions involved in cognition.
This field of research was redefined by the identification of
the AT(4) receptor as the trans membrane enzyme,
insulin-regulated membrane amino peptidase (IRAP). Here, we
explore the potential mechanisms by which Ang IV binding to
IRAP leads to the facilitation of learning and memory.
Albiston AL, et al. Trends
Endocrinol Metab 2003 Mar;14(2):72-7
Although
angiotensin II has long been considered to represent the end
product of the renin-angiotensin system (RAS), there is
accumulating evidence that it encompasses additional effector
peptides with diverse functions. In this respect, angiotensin
IV (Ang IV) formed by deletion of the two N terminal amino
acids, has sparked great interest because of its wide range of
physiological effects. Among those, its facilitatory role in
memory acquisition and retrieval is of special therapeutic
relevance. High affinity binding sites for this peptide have
been denoted as AT(4)- receptors and, very recently, they have
been proposed to correspond to the membrane-associated OTase/
IRAP amino peptidase. This offers new opportunities for
examining physiological roles of Ang IV in the fields of
cognition, cardiovascular and renal metabolism and
pathophysiological conditions like diabetes and hypertension.
Still new recognition sites may be unveiled for this and other
angiotensin fragments. Recognition sites for Ang-(1-7)
(deletion of the C terminal amino acid) are still elusive and
some of the actions of angiotensin III (deletion of the N
terminal amino acid) in the CNS are hard to explain on the
basis of their interaction with AT(1)-receptors only. A more
thorough cross-talk between in vitro investigations on native
and transfected cell lines and in vivo investigations on
healthy, diseased and transgenic animals may prove to be
essential to further unravel the molecular basis of the
physiological actions of these small endogenous angiotensin
fragments.
Vauquelin G, et al. J
Renin Angiotensin Aldosterone Syst 2002 Dec;3(4):195-204
The role of angiotensin IV (Ang IV) in the
regulation of angiotensin-converting enzyme (ACE) was studied
in vitro. This study demonstrates that this active fragment
appeared as a novel endogenous ACE inhibitor. Inhibitory
kinetic studies revealed that Ang IV acts as a purely
competitive inhibitor with a K(i) value of 35 microM. Ang IV
was found to be quite resistant to ACE hydrolysis opposite to
hemorphins which are both ACE inhibitors and substrates. In
order to confirm a putative role of Ang IV and hemorphins in
the Renin-Angiotensin system (RAS) regulation, we studied
their influence on Ang I conversion. We noticed that 16.7
microM of both peptides decreased more than 50% of Ang I
conversion to Ang II in vitro. The capacity of hemorphins,
particularly LVVH-7, and Ang IV to inhibit ACE activity here
suggests a synergistic relation between these two peptides and
the regulation of RAS.
Fruitier-Arnaudin I, et al. Peptides 2002
Aug;23(8):1465-70
Biosynthetic pathways for the
formation of neuroactive peptides and the processes for their
inactivation include several enzymatic steps. In addition to
enzymatic processing and degradation, several neuropeptide's
have been shown to undergo enzymatic conversion to fragments
with retained or modified biological activity. This has most
clearly been demonstrated for e.g. opioid peptides,
tachykinins, calcitonin gene-related peptide (CGRP) as well as
for peptides belonging to the renin-angiotensin system.
Sometimes the released fragment shares the activity of the
parent compound. However, in many cases the conversion
reaction is linked to a change in the receptor activation
profile, i.e. the generated fragment acts on and stimulates a
receptor not recognized by the parent peptide. This review
will describe the characteristics of certain neuropeptide
fragments having the ability to modify the biological action
of the peptide from which they are derived. Focus will be
directed to the tachykinins, the opioid peptides,
angiotensin's as well as to CGRP, bradykinin and nociceptin.
The kappa opioid receptor selective opioid peptide, dynorphin,
recognized for its ability to produce dysphoria, is converted
to the delta opioid receptor agonist Leu-enkephalin, with
euphoric properties. The tachykinins, typified by substance P
(SP), is converted to the bioactive fragment SP(1-7), a
heptapeptide mimicking some but opposing other effects of the
parent peptide. The bioactive angiotensin II, known to bind
to and stimulate the AT-1 and AT-2 receptors, is converted to
angiotensin IV (i.e. angiotensin 3-8) with preference for the
AT-4 sites or to angiotensin (1-7), not recognized by any of
these receptors. Both angiotensin IV and angiotensin (1-7) are
biologically active. For example angiotensin (1-7) retains
some of the actions ascribed for angiotensin II but is shown
to counteract others. Thus, it is obvious that the
activity of many neuroactive peptides is modulated by
bioactive fragments, which are formed by the action of a
variety of peptidases. This phenomenon appears to represent an
important regulatory mechanism that modulates many
neuropeptide systems but is generally not acknowledged.
Hallberg M, Nyberg F. Curr Protein
Pept Sci 2003 Feb.;4(1):31-44
The role of angiotensin II (AII) and
angiotensin IV (AIV) as inducers of PAI-1 expression during
hypertension was studied in vivo. A 2-week infusion of AII
(300 ng/kg/min) via an osmotic pump increased systolic blood
pressure (171 2 vs. 138 6 mm Hg), urinary protein excretion
(32 6 vs. 14 2 mg/day), and renal (2.2 0.5 vs. 1.0 0.1) and
cardiac (1.8 0.3 vs. 1.0 0.1) gene expression of plasminogen
activator inhibitor 1 (PAI-1). AIV infusion did not affect any
of the above with the exception of PAI-1 gene expression which
was increased in the left ventricles (1.7 0.3 vs. 1.0 0.1).
AII-infused rats displayed a decreased creatinine clearance
(538 75 vs. 898 96 ml/min) and hypertrophic left ventricles
(0.275 0.006 vs. 0.220 0.011 g/100 g). Our results demonstrate
that AII but not AIV infusion is associated with increased
renal PAI-1 gene expression.
Abrahamsen CT, et al. Pharmacology 2002
Sep;66(1):26-30
The octapeptide hormone,
angiotensin II (Ang II), exerts its major physiological
effects by activating AT(1) receptors. In vivo Ang II is
degraded to bioactive peptides, including Ang III
(angiotensin-(2-8)) and Ang IV (angiotensin-(3-8)). These
peptides stimulate inositol phosphate generation in human
AT(1) receptor expressing CHO-K1 cells, but the potency of Ang
IV is very low. Substitution of Asn(111) with glycine, which
is known to cause constitutive receptor activation by
disrupting its interaction with the seventh trans membrane
helix (TM VII), selectively increased the potency of Ang IV
(900-fold) and angiotensin-(4-8), and leads to partial agonism
of angiotensin-(5-8). Consistent with the need for the
interaction between Arg(2) of Ang II and Ang III with
Asp(281), substitution of this residue with alanine (D281A)
decreased the peptide's potency without affecting that of Ang
IV. All effects of the D281A mutation were superseded by the
N111G mutation. The increased affinity of Ang IV to the N111G
mutant was also demonstrated by binding studies. A model is
proposed in which the Arg(2)-Asp(281) interaction causes a
conformational change in TM VII of the receptor, which,
similar to the N111G mutation, eliminates the constraining
intra molecular interaction between Asn(111) and TM VII. The
receptor adopts a more relaxed conformation, allowing the
binding of the C-terminal five residues of Ang II that
switches this "pre activated" receptor into the fully active
conformation.
Le MT, et al. J Biol
Chem 2002 Jun 28;277(26):23107-10
OBJECTIVE: The aim of the present study
was to investigate whether angiotensin II (Ang II),
angiotensin III (Ang III) or Ang II (2-8), angiotensin IV (Ang
IV) or Ang II (3-8) and Ang II (1-7), Ang II (4-8), Ang II
(5-8) and Ang II (1-4) can stimulate collagen gel contraction
in cardiac fibroblasts in serum-free conditions. METHODS:
Cardiac fibroblasts (from male adult Wistar rats) from passage
2 were cultured to confluency and added to a hydrated collagen
gel in a Dulbecco's Modified Eagle's Medium, with or without
foetal bovine serum, for one, two or three days. The area of
the collagen gels embedded with cardiac fibroblasts was
determined by a densitometric analysis. Collagen gel
contraction was characterized by a decrease in the gel area.
RESULTS: Ang II dose-dependently stimulated the contraction of
collagen mediated by cardiac fibroblasts after one, two or
three days of incubation in a serum-free medium. Telmisartan
completely blocked the Ang II-induced collagen contraction by
cardiac fibroblasts. PD 123319 and des-Asp(1)-Ile(8)-Ang II
had no effect on the Ang II-induced collagen contraction by
cardiac fibroblasts. Ang III also stimulated the contraction
of collagen mediated by cardiac fibroblasts after one, two or
three days of incubation in a serum-free medium.
des-Asp(1)-Ile(8)-Ang II and telmisartan completely blocked
the Ang III-induced collagen gel contraction by cardiac
fibroblasts. des-Asp(1)-Ile(8)-Ang II, however, had no effect
on the Ang II-induced collagen gel contraction by cardiac
fibroblasts. Ang IV and Ang II (4-8), (5-8), (1-7) and (1-4),
however, had no effect on collagen gel contraction by cardiac
fibroblasts. Addition of telmisartan, PD 123319 or
des-Asp(1)-Ile(8)-Ang II alone did not affect collagen gel
contraction by cardiac fibroblasts. CONCLUSION: Our data
demonstrate that the effects of Ang II on the collagen gel
contraction by adult rat cardiac fibroblasts in serum-free
conditions are Ang II type 1(AT(1))-receptor- mediated,
because they are abolished by the specific AT(1)-receptor
antagonist, telmisartan, and not by the AT(2)-receptor
antagonist PD 123319 or by the Ang III antagonist
des-Asp(1)-Ile(8)-angiotensin. The Ang III- stimulated
contraction of collagen by cardiac fibroblasts is completely
blocked by the Ang III receptor antagonist,
des-Asp(1)-Ile(8)-angiotensin II, and by
telmisartan.
Lijnen P, et al. J
Renin Angiotensin Aldosterone Syst 2002 Sep;3(3):160-6
Trunk blood was obtained from 8-wk-old
salt-replete SHR (n = 8) or salt-depleted
SHR (n = 8) for determination of plasma
concentrations of angiotensin peptides by RIA . Blood was collected
into chilled Vacutainer tubes containing a mixture of
peptidase inhibitors: 25 mM EDTA, 0.44 mM
1,20-orthophenanthrolene monohydrate (Sigma, St. Louis, MO),
1 mM sodium parachloromercuribenzoate, and 3 µM WFML (rat renin inhibitor:
acetyl-His-Pro-Phe-Val-Statine-Leu-Phe). After
20 min on ice, blood samples were centrifuged at
3,000 rpm for 20 min, and aliquots of plasma were
stored at -80°C until assayed for angiotensin peptides. Plasma was extracted on a
Sep-Pak C18 column according to our previously published
protocol . The sample was eluted, reconstituted, and split for
the RIA of ANG I, ANG II.
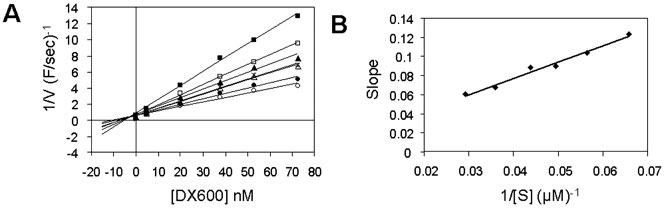
Ki determination of DX600 peptide using ACE2 assays with the
synthetic substrate. DX600, at concentrations
ranging from 5 to 73 nM, was pre incubated
with 7 nM ACE2. The substrate M-2195 was added at
concentrations ranging from 12 to 50 µM.
A, Dixon plot. Filled squares,
12.6 µM substrate (M-2195); open squares,
15.2 µM; filled triangles, 17.7 µM;
×, 20.2 µM; open triangles, 22.8 µM; filled circles, 27.8 µM; and open
circles, 45.6 µM.
B, Dixon secondary
plot. The slope at each substrate concentration in A was plotted against the reciprocal substrate
concentration. Data were fitted to a linear regression
(y = mx + b,
where m = Km/(Ki × Vmax) = 1.7077, b = 0.0089). Km (20.6 µM) and Vmax
(4.3 farads/s) were obtained by a fit of the data
in the absence of inhibitor to the Michaelis-Menten
equation by nonlinear regression analysis. Ki was calculated to be 2.8 nM
from the equation Ki = Km/(Vmax × m).
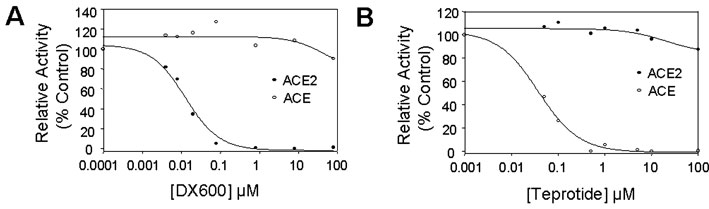
DX600 was a specific
inhibitor to ACE2. A,
effects of DX600 on ACE2 and ACE activity. Increasing
concentrations of DX600 peptides were incubated with
ACE2 (20 nM) or ACE (7.5 nM) prior to the
addition of the substrate M-2195 (50 µM). B, effects of ACE peptide inhibitor teprotide
on ACE2 and ACE activity. Increasing concentrations of
teprotide were incubated with ACE2 (20 nM) or ACE
(7.5 nM) prior to the addition of the substrate
M-2195. The relative enzymatic activity was plotted
against the peptide concentration. ACE2, filled
circle; ACE, open circle.
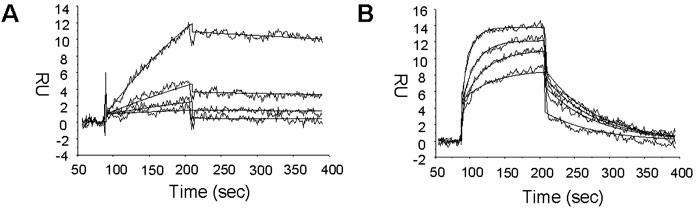
Kd determination of DX600 and DX512 peptides. The
binding affinities of the very strong inhibitors were
analyzed by BIAcore as described under "Experimental
Procedures." Shown here are representative sensor grams
of DX600 (A) and DX512 (B). The data
(response units, RU) were background corrected
and plotted against time (seconds). The wavy
lines depict actual data, and the solid
lines depict fitted data. DX600 was assayed at
100, 50, 25, and 12.5 nM,
corresponding to respective curves from top to bottom. DX512 was assayed at
500, 250, 125, and 62.5 nM,
corresponding to respective curves from top to bottom.
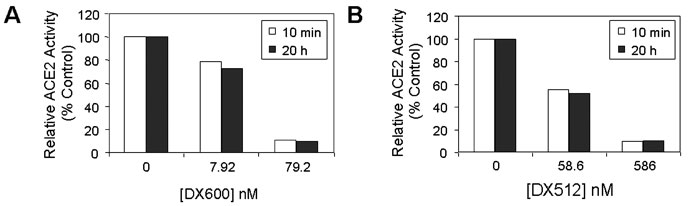
DX600 and DX512 peptides were stable ACE2 inhibitors. DX600 (A) and DX512
(B), at both low and high concentrations, were
each incubated with ACE2 (20 nM) for 10 min or
20 h at room temperature prior to the addition of
M-2195. The relative ACE2 activity was plotted against
the peptide concentration. Open square,
10 min; filled square, 20 h.
Huang L.
L., et al. J. Biol. Chem., Vol. 278, Issue 18,
15532-15540, May 2, 2003
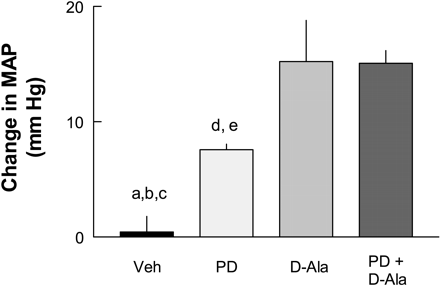
Maximal change in blood pressure produced by 5%
dextrose in water (Veh), PD-123319, [D-Ala7]ANG-(1-7),
or the combination of PD-123319 and [D-Ala7]ANG-(1-7) in
AT1-blocked, salt-depleted SHR. PD-123319 was infused at
0.12 µmol/min, and [D-Ala7]ANG-(1-7) was infused at
10 pmol/min. To assess the effect of fluid infused,
Veh was infused at 0.2 ml/min. Individual drugs
were infused at a rate of 0.1 ml/min; combined drug
infusion such as PD-123319 and [D-Ala7]ANG-(1-7)
amounted to 0.2 ml/min. a P < 0.05 for Veh vs.
PD-123319; b P < 0.05 for Veh
vs. [D-Ala7]ANG-(1-7); c P < 0.05 for Veh vs.
PD-123319 + [D-Ala7]ANG-(1-7); d P < 0.05 for PD-123319 vs.
[D-Ala7]ANG-(1-7); e P < 0.05 for PD-123319 vs.
PD-123319 + [D-Ala7]ANG-(1-7).
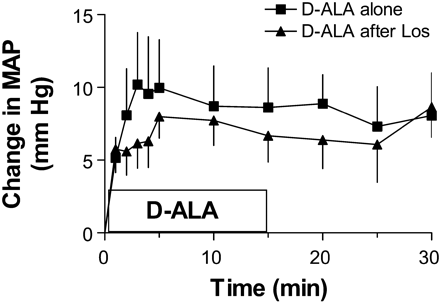
Effect of [D-Ala7]ANG-(1-7) infusion in
salt-depleted SHR in the absence and presence of AT1
receptor blockade. [D-Ala7]ANG-(1-7) infusion in
conscious salt-depleted SHR rapidly increased mean
arterial pressure (MAP) irrespective of whether AT1
receptors had been previously blocked by losartan
(32.5 µmol/kg iv).
Nakamura S., et al. Am J
Physiology Regul Integr Comp Physiology 284:
R164-R173, 2003
|
|
|
angiotensin;BKNew
%002-26%;%002-18%
|
|
|


