|
|
|
Orexins (Hypocretins) - Brain-Gut Peptides |
A family of hypothalamic neuropeptides and GPCR that regulate feeding behavior
|
Orexin-A and B were identified as endogenous ligands for the orexin-1 (HCRT1) and orexin-2 (HCRT2) G-protein coupled receptors (Sakurai et al, 1998). These peptides are identical to two hypothalamic peptides that share a high degree of homology with secretin, designated hypocretin-1 and hypocretin-2 (De Lecea et al, 1998). ICV administration of orexin-A and B stimulated food intake in rats. Additionally, prepro-orexin mRNA levels were upregulated in fasting rats. Recent reports suggest a role in the sleep-wake cycle.
Korczynski et al. J Physiol Pharmacol. 2006 Nov;57 Suppl 6:17-42.
Nowak et al. Life Sci. 2000;66(5):449-54.
Orexin A and orexin B were initially identified as endogenous ligands for two orphan G protein-coupled receptors [104]. They were initially recognized as regulators of feeding behavior in view of their exclusive production in the lateral hypothalamic area (LHA), a region known as the feeding center, and their pharmacological activity [104,30,49,107]. Subsequently, the finding that orexin deficiency causes narcolepsy in humans and animals suggested that these hypothalamic neuropeptides play a critical role in regulating sleep/wake cycle [22,46,71,95,117]. These peptides activate waking-active monoaminergic and cholinergic neurons in the hypothalamus/brain stem regions to maintain a long, consolidated awake period. Recent studies on efferent and afferent systems of orexin neurons, and phenotypic characterization of genetically modified mice in the orexin system further suggested roles of orexin in the coordination of emotion, energy homeostasis, reward system, and arousal [3,80,106,137]. A link between the limbic system and orexin neurons might be important for increasing vigilance during emotional stimuli. Orexin neurons are also regulated by peripheral metabolic cues, including ghrelin, leptin, and glucose, suggesting that they might have important roles as a link between energy homeostasis and vigilance states [137]. Recent research has also implicated orexins in reward systems and the mechanisms of drug addiction [13,48,91]. These observations suggest that orexin neurons sense the outer and inner environment of the body, and maintain proper wakefulness of animals for survival. This review discusses the mechanism by which orexins maintain sleep/wakefulness states, and how this mechanism relates to other systems that regulate emotion, reward, and energy homeostasis.
Ohno et al. Front Neuroendocrinol. 2008 Jan;29(1):70-87.
Orexin-A-like immunoreactivity was detected in every region of human brain, but not in the pituitary. The highest concentration of orexin-A-like immunoreactivity in the human brain was found in hypothalamus (17.8 +/- 4.3 pmol/g wet weight, mean +/- SEM, n = 7), followed by thalamus, medulla oblongata, and pons. Orexin-A-like immunoreactivity was detected in the tumor tissues of ganglioneuroblastoma and neuroblastoma, but not in the tumor tissues of pheochromocytoma. Reverse phase high performance liquid chromatographic analyses of the orexin-A-like immunoreactivity in the human brain extracts and neuroblastoma extracts showed a single immunoreactive peak, which was eluted in an identical position to synthetic human orexin-A.
Arihara Z, et al., Peptides 2000 Apr;21(4):565-70
RECENT STUDIES USING MOLECULAR GENETICS IN MICE AND DOGS, AS WELL AS HISTOPATHOLOGICAL ANALYSES OF HUMAN DISEASE, HAVE COME TO THE SAME CONCLUSION: the human sleep disorder narcolepsy is caused by failure of signaling mediated by orexin (hypocretin) neuropeptides. These and other findings strongly suggest that the orexin system plays a critical role in sleep/wake regulation. In addition, the orexin system may link energy homeostasis to the regulation of sleep/wake cycles.
Mieda M, Yanagisawa M. Curr Opin Neurobiol 2002 Jun;12(3):339-45
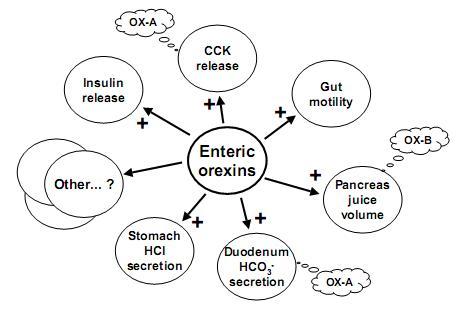
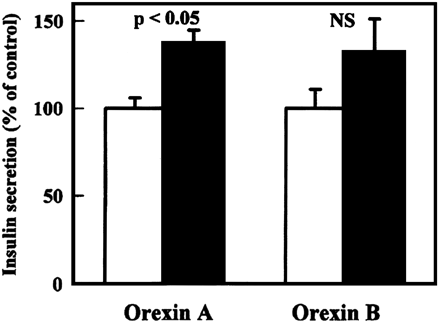
Orexins (hypocretins) are a novel pair of neuropeptides implicated in the regulation of energy balances and arousal. Previous reports have indicated that orexins are produced only in the lateral hypothalamic area, although orexin-containing nerve fibers were observed throughout the neuroaxis. Recent evidence shows that orexins and functional orexin receptors are found in the periphery. Vagal and spinal primary afferent neurons, enteric neurons, and endocrine cells in both the gut and pancreas display orexin- and orexin receptor-like immunoreactivity. Orexins excite secretomotor neurons in the guinea pig gut and modulate gastric and intestinal motility and secretion. In addition, orexins modulate hormone release from pancreatic endocrine cells. Moreover, fasting up-regulates the phosphorylated form of cAMP response element binding protein in orexin-immunoreactive enteric neurons, indicating a functional response to food status in these cells. The purpose of this article is to summarize evidence for the existence of a brain-gut network of orexin-containing cells that appears to play a role in the acute regulation of energy homeostasis.
Kirchgessner. Endocr Rev. 2002 Feb;23(1):1-15.
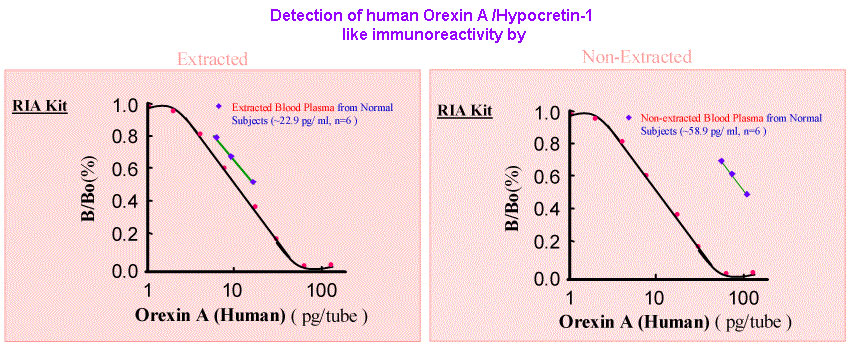
Orexin-A and -B stimulate appetite and food intake in rats. Orexins and orexin receptors are present in the hypothalamus as well as the enteric nervous system, the pancreas and the gut. The presence of orexins in peripheral blood, however, has not yet been reported. To determine whether orexin-A is present in human plasma and is related to body weight, we measured plasma orexin-A and leptin levels in a population with a body mass index (BMI) range from 19.8 to 59 kg/m(2). Plasma orexin-A levels correlated negatively and plasma leptin levels correlated positively with BMI. In obese and morbidly obese individuals, orexin-A levels were significantly lower and leptin levels were significantly higher when compared to normal. Our results support previous data suggesting that orexin-A acts also in a peripheral manner. The fact that lower levels of plasma orexin-A are present in obese individuals suggests that it is involved in the regulation of human energy metabolism.
Adam JA, et al. Int J Obes Relat Metab Disord 2002 Feb;26(2):274-6
Orexins are neuropeptides that are localized in neurons within the lateral hypothalamus and regulate feeding behavior. The lateral hypothalamus plays an important role in not only feeding but also in the central regulation of gut function. Along this line, accumulating evidence has shown that orexins act in the central nervous system to regulate gastrointestinal functions. The purpose of this review is to summarize recent relevant findings on brain orexins and the digestive system, and discuss the pathophysiological roles of these peptides. Centrally administered orexin or endogenously released orexin in the brain potently stimulates gastric acid secretion in rats. The vagal cholinergic pathway is involved in the orexin-induced stimulation of acid secretion. Because of its stimulatory action on feeding, it can be hypothesized that orexin in the brain is a candidate mediator of cephalic phase gastric secretion. In addition, brain orexin may be involved in the development of depression and functional gastrointestinal disorders, which are frequently accompanied by inhibition of gut function, because lack of orexin activity might cause the inhibition of gastric physiological processes and evoke a depressive state. These lines of evidence suggest that orexin in the brain is a potential molecular target for treatment of functional gastrointestinal disorders.
Okumura et al. J Gastroenterol. 2008;43(9):652-60.
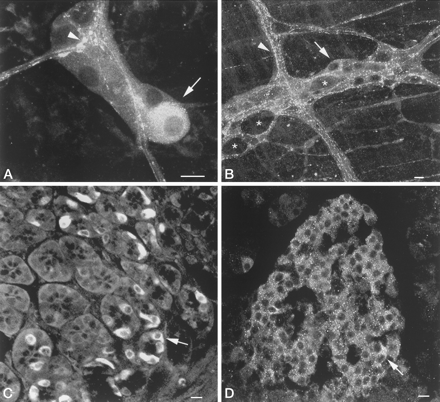
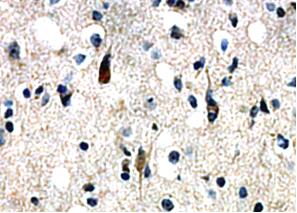
Orexin-A immunoreactivity in the guinea pig gut and pancreas.
A, Submucosal ganglion. Orexin-A immunoreactivity is displayed
by a subset of cell bodies (arrow) and varicosities (arrowhead).
B, Myenteric ganglion. Orexin-A immunoreactivity is displayed by
a subset of cell bodies (arrow) and varicose nerve fibers in
interganglionic connectives (arrowhead) and surrounding unlabeled
cell bodies (*). C, Orexin-A immunoreactive endocrine cells
(arrow) in the gastric mucosa. D, Orexin-A immunoreactive cells
(arrow) in a pancreatic islet. Orexin-A-containing cells are
present throughout the islet. Scale bars, 50 µm (A and B), 10 µm (C and D).
Kirchgessner. Endocr Rev. 2002 Feb;23(1):1-15.
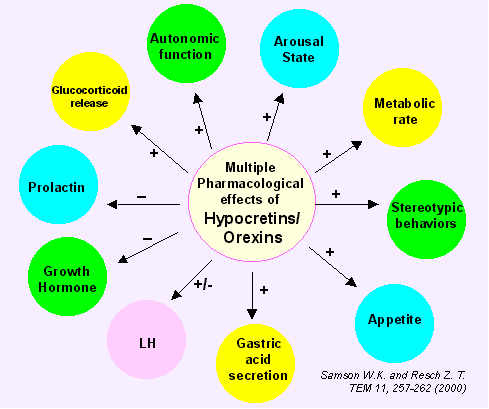
Maintenance of energy homeostasis requires the coordination of systems that regulate feeding, body temperature, autonomic and endocrine functions with those that govern an appropriate state of arousal (wakefulness). Historically, the hypothalamus has been recognized to play a critical role in maintaining energy homeostasis by integrating these factors and coordinating metabolic, neuroendocrine and behavioral responses and arousal states. Recent studies have suggested that orexin-containing neurons in the lateral hypothalamic area (LHA) constitute an important central pathway that promotes adaptive behavioral and arousal responses to metabolic and environmental signals. Orexins, also called hypocretins, are neuropeptides originally identified as endogenous ligands for two orphan G-protein-coupled receptors termed orexin receptors -1 and -2. Orexin-A and -B are expressed by a specific population of neurons in the LHA. These neurons project to numerous brain regions, with monoaminergic and cholinergic nuclei of the hypothalamus, midbrain, and pons receiving particularly strong innervations. The orexinergic system is anatomically well-placed to coordinate the metabolic, motivational, motor, autonomic, and arousal processes necessary to elicit environmentally appropriate behaviors. Recent studies on orexin suggest that the orexinergic system plays a significant role in feeding and sleep-wakefulness regulation, possibly by coordinating the complex behavioral and physiological responses of these complementary homeostatic functions. Orexin neurons may provide an integrative link between peripheral metabolism and central regulation of behaviors required for an adaptive response to homeostatic challenges.
Sakurai T. Neuroreport 2002 Jun 12;13(8):987-95
The concentrations of immunoreactive α-MSH was quantitated in thePVN, using commercially available 125 I RIA kit [α-MSH(acetylated a-MSH); Phoenix Pharmaceuticals]. For extrac-tionof PVN peptides, 1 ml of 0.1 M acetic acid was added toeach tissue sample, which was then transferred to a boilingwater bath for 10 min. After cooling on ice, samples werehomogenized in polypropylene tubes. The homogenates werecentrifuged at 13,000 g for 15 min. The pellet was resuspended in 3 N NaOH and analyzed for total protein content. To assay for protein, 150 µl of supernatant were taken fromeach sample, and the remainder of supernatant was lyophilizedand later used for α-MSH RIA. The RIA kit was validated before use with tissue extracts.Dose-response curves for PVN tissue extract, and increasing concentrations of the a-MSH added to a rat PVN tissue extract were parallel (P < 0.05) tothe standard curve. a-MSH (rangingfrom 1 to 32 pg) added to rat PVN tissue extract was consistently recovered from 100 µl of extract (90-100%). The assay sensitivity was 4 pg/tube. The cross-reactivity test, pro-videdby Phoenix Pharmaceuticals, indicated 0% cross-reactivity with opioid peptides, 0% cross-reactivity with β- and γ-MSH, and 0.02% cross-reactivity with ACTH for α-MSH assay.
Kim, Eun-Mee, et al., Physiol. 276: R1320-R1326, 1999.

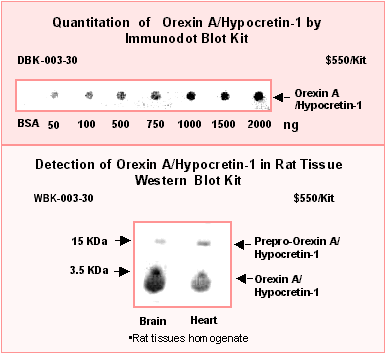
Using Phoenix Pharmaceuticals' Hypocretin-1 / Orexin A RIA kit, Nishino, et al. have found that Hypocretin/Orexin-like immunoreactivity in the cerebrospinal fuid (CSF) from narcolepsy patients is under detectable levels [1] as compared with normal subjects (<40 ng/ml vs 285 ng/ml).
The hypothalamus is a major regulatory center for autonomic and endocrine homeostasis, were hypothalamic-specific peptides exert their important roles by transport to the pituitary, by entering the general circulation, or by secrection within the central nervous system. Hypocretins/Orexins are hypothalamic- specific peptides with neuro-excitatory, feeding behavior, endocrine and cardiovascular function [4,7,8,9,10,11]. Recently, attention has been focused on their role in sleep regulation [1,2,3,5,6]. Human narcolelpsy may relate to Hypocretin / Orexin deficiency in brain.
Neurons containing hypocretin are located exclusively in the lateral hypothalamus and send axons to mumerous regions throughout the central nervous system, including the major muclei implicated in sleep control. It was reported that the brain region receiving the densest innervation from orexinergic nerves is the locus coeruleus, a key modulator of attentional state, where application of Orexin A increased cell firing of intrinsic noradrenergic neurons. Orexin A increased arousal and locomotor activity and modulated neuroendocrine functions[5]. These data suggest that Hypocretins/Orexins play an important role in orchestrating the sleep-wake cycle.
Narcolepsy is a disabling sleep disorder affecting humans and animals. It is characterized by daytime sleepiness, cataplexy, and striking trasitions from wakefulness into rapid eye movement (REM) sleep [2]. Lin, et al. have found that canine narcolepsy can be caused by disruption of the Hypocretin/Orexin receptor 2 gene (Hcrtr2)[2]. Hornozygous Hypocretin knock out mice showed a phenotype strikingly similar to that in human narcolepsy patients, as well as canarc-1 mutant dogs, the only known monogenic model of narcolepsy [3].
This is the first report that Hypocretin neurotransmission is deficient in some people with narcolepsy. These results, together with the observation that hypocretin receptor and peptide gene alterations induce narcolepsy in animal models, suggest that Hypocretin/Orexin deficiency contributes to narcolepsy.
- Nishino, S. et al. Hypocretin (Orexin) deficiency in human narcolepsy. Lancet, 355, 39-40 (2000)
- Lin, L. et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell, 98, 365-376 (1999)
- Chemelli, R.M., et al/ Narcolepsy in Orexin knock out mice: molecular genetics of sleep regulation. Cell 98, 437-451 (1999)
- De Lecea, L. et al. The Hypocretins hypothalamous-specific peptides with neuroexcitatory activity. Proc. Nat. Acad. Sci. 95, 322-327 (1998)
- Hagan, J.J. et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Nat. Acad. Sci. 96, 10911-10916 (1999)
- Siegel, J.M. Narcolepsy, a key role for Hypocretins (Orexins). Cell 98, 409-412 (1999)
- Samson, W.K., Gosnell, B., Chang, J.K., et al. Cardiovascular regulatory actions of the Hypocretins in brain. Brain Res. 831, 241-253 (1999)
- Kirchgessner, A.L. and Liu, M. Orexin synthesis and response in the gut. Neuron 1999 Dec. 24 (4): 941-51
- Lopez, M. et al. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology 140, 5991-4 (1999)
- Yamamoto, Y. et al. Orexin receptors are expressed in the adreanal medulla of the rat. Brain Res Mol Brain Res. 65, 14-22 (1999)
- Antonarakis, S.E. and Mckusick V.A. OMIM *602393 Hypocretin Receptor
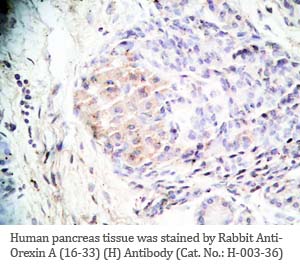 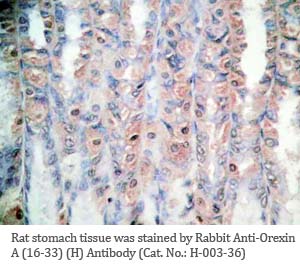
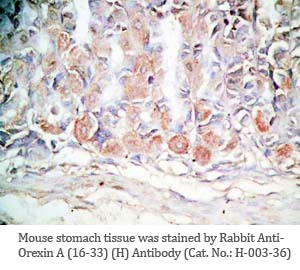 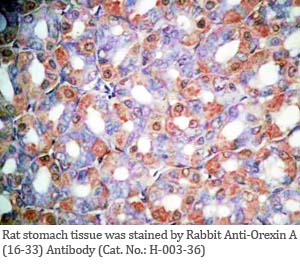
Tissue Sample |
Human pancreas, rat/mouse stomach tissues |
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody |
Pretreatment |
N/A |
Blocking |
3% H2O2, 2% Normal Goat Serum |
Primary Antibody |
rabbit anti-Orexin A (16-33) (Human, Rat) antibody (Cat. No.: H-003-36) |
Optimal Dilution |
1: 500 |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
Streptavidin-HRP (Vector), 1:400, 30 min |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
 |
| Orexin
B(3-28) |
100 |
| Orexin
B(1-28) |
100 |
| Orexin
B (Human) |
100 |
| Orexin
A |
0 |
| Leptin
(Human) |
0 |
| AGRP(83-132)(Human)-NH2 |
0 |
| AGRP(82-131)(Mouse) |
0 |
| NPY |
0 |
| MCH
(Rat) |
0 |
| MGOP27 |
0 |
| NEI |
0 |
|
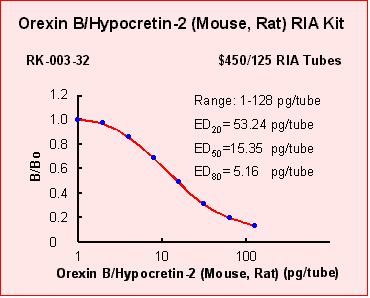 |
| Orexin B (3-28) (Mouse, Rat) |
100 |
| Orexin A (Human) |
100 |
| AGRP(83-132)(Human)-NH2 |
100 |
| AGRP(82-131)(Mouse) |
0 |
| Leptin
(Human) |
0 |
| MCH
(Rat) |
0 |
| MGOP27 |
0 |
| α-MSH |
0 |
| NEI |
0 |
|
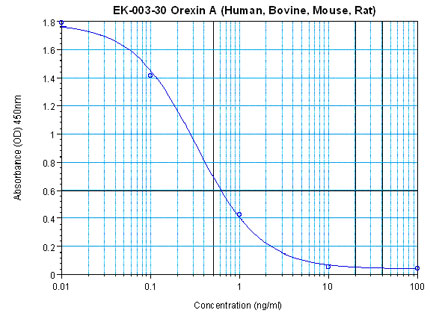
Linear Range: 0.22-2.28 ng/ml
| 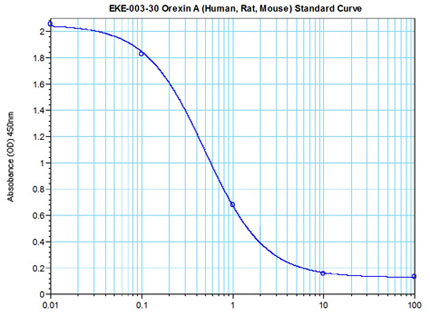
Linear Range: 0.19-1.68ng/ml
|
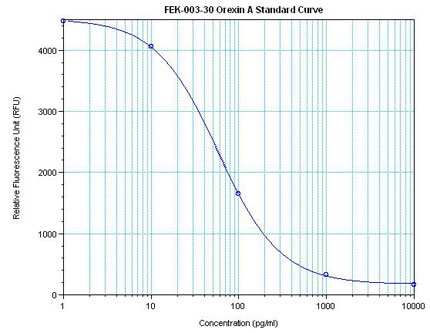
Linear Range: 42.5-480 pg/ml
5 times more sensitive than normal EIA Kit
|
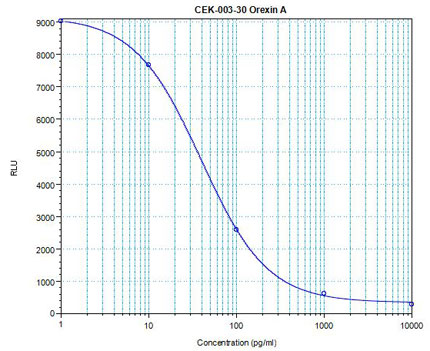 Linear Range: 12.2 - 156 pg/ml Linear Range: 12.2 - 156 pg/ml
18 times more sensitive than normal EIA Kit
|
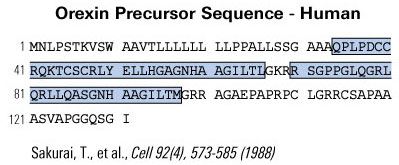
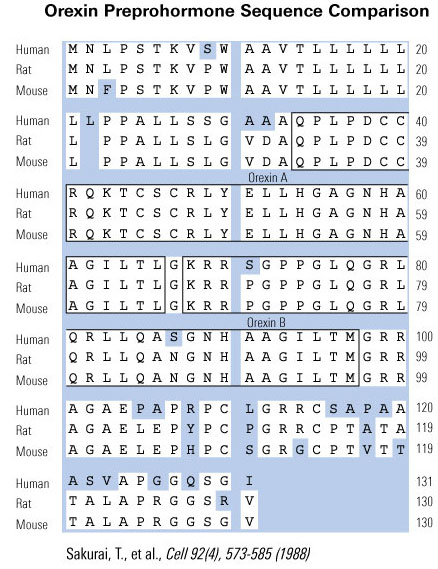
Difference in Hypocretin-1 (Hyp-1) and Orexin-A.
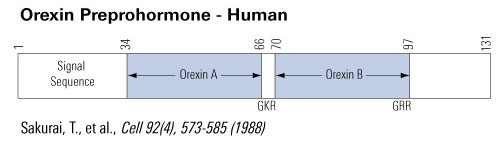
The amino acid sequence of Hyp-1 is identical to that of Orexin-A except Hyp-1 has 5 additional amino acids at the N-terminal (Leu-Gly-Val-Asp-Ala). Both are amidated at the C-terminal.
Difference in Hypocretin-2 (Hyp-2) and Orexin-B.
The amino acid sequence of Hyp-2 is identical to that of Orexin-B, including an amidated at the C-terminal. p
De Lecea et al. Proc Natl Acad Sci U S A. 1998 Jan 6;95(1):322-7.
Sakurai et al. Cell. 1998 Feb 20;92(4):573-85.

- Pressor effects of orexins injected intracisternally and to rostral
ventrolateral medulla of anesthetized rats. Chen CT, Hwang LL, Chang JK, Dun NJ. Am J Physiol Regul Integr Comp Physiol 2000 Mar;278(3):R692-7
- Orexin A-like immunoreactivity in the rat brain.
Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Neurosci Lett 1999 Feb 5;260(3):161-4
- Orexins: a role in medullary sympathetic outflow.
Dun NJ, Le Dun S, Chen CT, Hwang LL, Kwok EH, Chang JK. Regul Pept 2000 Dec 22;96(1-2):65-70
- Hypothalamic orexin A-immunoreactive neurons project to the rat dorsal medulla.
Harrison TA, Chen CT, Dun NJ, Chang JK. Neurosci Lett 1999 Sep 24;273(1):17-20
- Cardiovascular regulatory actions of the hypocretins in brain.
Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Brain Res 1999 Jun 12;831(1-2):248-53
|
|
|
%orexin%
|
|
|


