Shimada, M. et al., Nature 396, 670-673 (1998)

Two groups have identified a 353 amino acid G-coupled orphan receptor as
receptor for MCH: Chambers, J. et al., Nature 400, 261-265 (1999) and Saito, Y.
et al., Nature 400, 265-269 (1999). We offer several rat SLC-1 receptor
fragments for antibody production as well as MCH and related peptides for
obesity research and other studies.
Melanin-concentrating hormone (MCH) and alpha-melanocyte-stimulating hormone (alpha-MSH) are known to exhibit mostly functionally antagonistic, but in some cases agonistic activities, e.g., in pigment cells and in the brain. Neuropeptide E-I (NEI) displays functional MCH-antagonist and MSH-agonist activity in different behavioral paradigms; the role of neuropeptide G-E (NGE) is not known. This study addressed the question of possible molecular interactions between alpha-MSH, MCH and the MCH-precursor-derived peptides NEI and NGE at the level of the pigment cell MCH receptor subtype (MCH-Rpc) and the different melanocortin (MC) receptors. Radioreceptor assays using [125I]MCH, [125l]alpha-MSH and [125I]NEI as radioligands and bioassays were performed with MCI-R-positive and MC1-R-negative mouse B16 melanoma cells and with COS cells expressing the different MC receptors. The IC50s of alpha-MSH and NEI or NGE for [125I]MCH displacement from mouse MCH-Rpc were 80-fold and, respectively, >300-fold higher than that of MCH, and the IC50s for MCH and NEI or NGE for [125I]alpha-MSH displacement from mouse MC1-R were 50,000-fold and >200,000-fold higher than that of alpha-MSH. No high-affinity binding sites for NEI were detected on B16 melanoma cells and there was no significant displacement of [1251]alpha-MSH by MCH, NEI or NGE with MC3-R, MC4-R and MC5-R expressed in COS cells. At concentrations of 100 nM to 10 microM, however, MCH, NEI and NGE induced cAMP formation and melanin synthesis which could be blocked by agouti protein or inhibitors of adenylate cyclase or protein kinase A. This shows that mammalian MCH-precursor-derived peptides may mimic MSH signalling via MC1-R activation at relatively high, but physiologically still relevant concentrations, as e.g. found in autocrine/paracrine signalling mechanisms.
Hintermann E, et al. J Recept Signal Transduct Res. 2001 Feb;21(1):93-116
The physiological role of melanin-concentrating hormone (MCH) in mammals is still very elusive, but this peptide might participate in the central control of the hypothalamopituitary adrenal (HPA) axis during adaptation to stress. Cloning and sequencing of the rat MCH (rMCH) cDNA revealed the existence of additional peptides encoded into the MCH precursor. Among these peptides, neuropeptide (N) glutamic acid (E) isoleucine (I) amide (NEI) is co-processed and secreted with MCH in rat hypothalamus. In the present work we examined: (1) The pattern of rMCH mRNA expression during the light and dark conditions in the rat hypothalamus and (2) The effect of intracerebroventricular (ICV) injections of rMCH and NEI in the control of basal or ether stress-modified release of corticotropin (ACTH), prolactin (PRL) and growth hormone (GH) secretion in vivo in light-on and light-off conditions. Our data indicate that rMCH mRNA levels do not change during the light-on period, but increase after the onset of darkness. Either alone or co-administered, rMCH and NEI do not modify basal secretion of GH and PRL at any time tested nor do they alter ether stress-induced changes in these two hormonal secretions. At the end of the light on period corresponding to the peak of the circadian rhythm in ACTH, administration of rMCH but not NEI leads to a decrease in ACTH levels while MCH is not effective during the light off period of the cycle (i.e. when basal ACTH levels are already low). Using a moderate ether induced stress, ACTH levels are only stimulated during the dark phase of the cycle.(ABSTRACT TRUNCATED AT 250 WORDS)
Bluet-Pajot MT, et al. J Neuroendocrinol. 1995 Apr;7(4):297-303
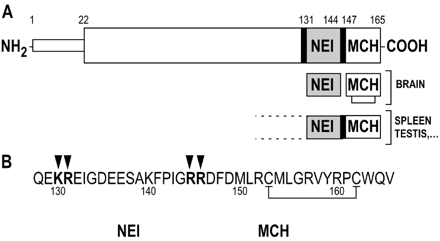
Melanin-concentrating hormone (MCH) is a cyclic neuropeptide, with a major
role in stimulation of feeding behavior in mammals. MCH signals in the brain
occur via two seven-transmembrane G protein-coupled receptors, namely MCH1
(SLC-1, MCH(1), MCH-R1, or MCH-1R) and MCH2 (SLT, MCH(2), MCH-R2, or MCH-2R).
In this study, we demonstrate that the pro-MCH(131-165) peptide
neuropeptide-glutamic acid-isoleucine (NEI)-MCH is more potent than MCH in
stimulating feeding in the rat. Using rat MCH1-expressed human embryonic kidney
293 cells, we show that NEI-MCH exhibits 5-fold less affinity in a binding
assay and 2-fold less potency in a cAMP assay than MCH. A similar 7- to 8-fold
shift in potency was observed in a Ca(2+)(i) assay using rat MCH1 or human
MCH2-transfected Chinese hamster ovary cell models. This demonstrates that
NEI-MCH is not a better agonist than MCH at either of the MCH receptors. Then,
we compared the proteolysis resistance of MCH and NEI-MCH to rat brain membrane
homogenates and purified proteases. Kinetics of peptide degradation using brain
extracts indicated a t(1/2) of 34.8 min for MCH and 78.5 min for NEI-MCH with a
specific pattern of cleavage of MCH but not NEI-MCH by exo- and endo-proteases.
Furthermore, MCH was found highly susceptible to degradation by aminopeptidase
M and endopeptidase 24.11, whereas NEI-MCH was fully resistant to proteolysis
by these enzymes. Therefore, our results strongly suggest that reduced
susceptibility to proteases of NEI-MCH compared with MCH account for its
enhanced activity in feeding behavior. NEI-MCH represents therefore the first
MCH natural functional "superagonist" so far described.

Changes in food intake after acute i.c.v. injection of MCH
and NEI-MCH. Either MCH or NEI-MCH was injected i.c.v. at time 0, and
cumulative food intake was measured over the following 6-h period in satiated
rats. Because of the large number of rats involved (n = 7), each dose of MCH,
NEI-MCH, and a control group was run on a separate day. The control groups
for each dose of peptide are shown as filled symbols and correspond to the
same convention as each dose of the peptides. Food intake for all groups was
analyzed by three-way analysis of variance (treatment × dose × time). Post
hoc test was by complementary analysis based on the Newman-Keuls test after
pooling all levels of time. , significantly different from the appropriate
control group; +, MCH and NEI-MCH are significantly different from each
other.
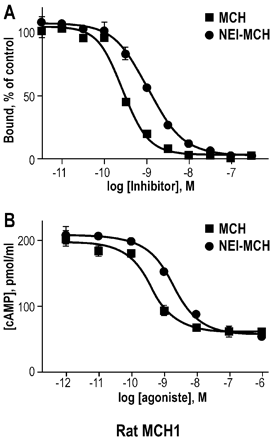
Binding affinity and functional activity of MCH and
NEI-MCH at the rat MCH1 receptor. Concentration-response effect of MCH and
NEI-MCH upon [125I]S36057-specific binding to membranes expressing the rat
MCH1 receptor (A) and upon the inhibition of forskolin-induced intracellular
cAMP level in cells stably expressing the rat MCH1 receptor (B). Points shown
are from a representative experiment performed in triplicate and repeated
independently at least three times.
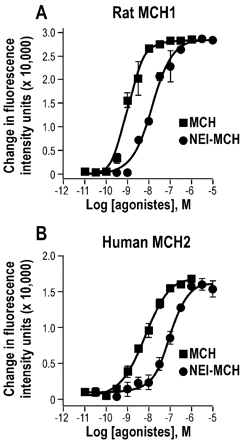
Effect of MCH and NEI-MCH upon intracellular calcium level
in cells expressing the rat MCH1 or the human MCH2 receptors.
Concentration-response effect of MCH and NEI-MCH upon intracellular calcium
in cells expressing the MCH1 (A) or the MCH2 (B) receptors. Results are
expressed as the mean percentage of the calcium peak height with the peak
height of 1 µM MCH taken as 100%. Points shown are from a representative
experiment performed in triplicate and repeated independently four times.

Kinetics of MCH and NEI-MCH degradation in rat brain
extracts. Peptides were incubated for up to 2 h at 37°C with brain extracts
(0.5 mg/ml protein concentration). Separation and quantification was
performed on RP-HPLC/UV as indicated under Materials and Methods. Exponential
equation was fitted to determine half-time and the initial velocity of the
peptide hydrolysis. Each point corresponds to the mean of three independent
experiments.
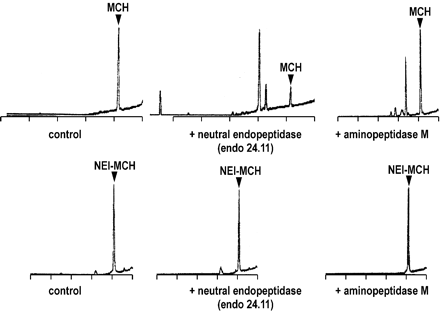
MCH and NEI-MCH hydrolysis by aminopeptidase M and
endopeptidase 24.11. MCH (top) or NEI-MCH (bottom) was incubated for 60 min
at 37°C in a final volume of 100 µl of Tris/HCl, pH 7.4, without protease
(control), or in the presence of 100 nM endopeptidase 24.11 or 200 nM
aminopeptidase M. Incubations were analyzed by RP-HPLC as described under
Materials and Methods.
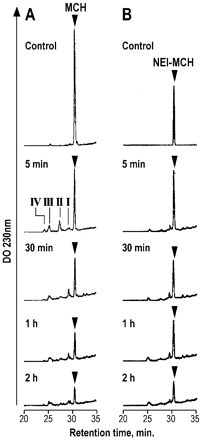
Time courses of MCH (left panel) and NEI-MCH (right panel)
hydrolysis by rat brain membranes. MCH or NEI-MCH (2 nmol) was incubated for
the indicated times at 37°C with 50 µg of brain extracts in a final volume of
100 µl of Tris/HCl, pH 7.4. Incubation mixtures were analyzed by RP-HPLC as
described under Materials and Methods. Arrowheads indicate the retention
times of MCH or NEI-MCH synthetic peptides. Roman numbers indicate the
peptide-containing peaks that were analyzed by MALDI-TOF mass spectrometry.
Maulon-Feraille L, J Pharmacol Exp Ther 2002 Aug;302(2):766-73
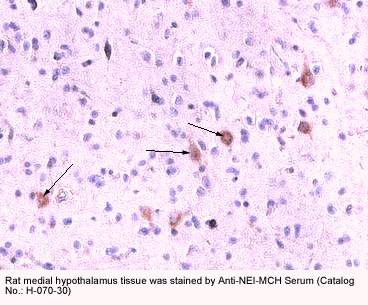
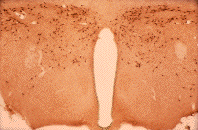
| 
|
|
4x | 40x |
Slides courtesy Dr. Nae Dun, James H. Quillen College of Medicine
East Tennessee State University
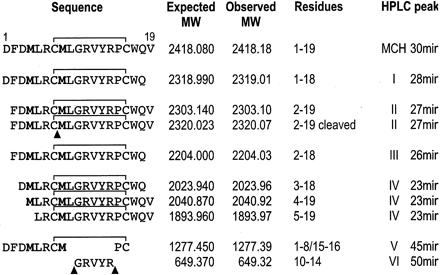
Sequence of MCH and metabolites identified by mass
assignments. Letters in bold indicate oxidized residues. Proposed sites of
cleavage by endopeptides are indicated by arrowheads. The degradation
products found in every HPLC peaks (numbered as in Fig. 6) were recovered and
analyzed by mass spectrometry as described under Materials and Methods.
Maulon-Feraille L, J Pharmacol Exp Ther 2002 Aug;302(2):766-73