|
|
|
FGF-21 - Fibroblast Growth Factor 21 |
OBJECTIVE: Reports of increased circulating fibroblast growth factor 21 (FGF21) levels in obesity indicate that FGF21 may be implicated in body weight homeostasis. We sought to investigate the existence of FGF21 in human cerebrospinal fluid (CSF) and, if present, the relationship between CSF FGF21 with body adiposity and metabolic parameters.
RESEARCH DESIGN AND METHODS: CSF and corresponding plasma FGF21 were measured by an enzyme-linked immunosorbent assay (18 men and 20 women, aged 19-80 years, and BMI 16.2-38.1 kg/m(2)) and correlated to body adiposity and metabolic parameters.
RESULTS: CSF and plasma FGF21 increased in particular with rising BMI and fat mass. In CSF, FGF21 was detectable at concentrations ~40% that of plasma levels. CSF and plasma FGF21 levels were significantly positively correlated with BMI and fat mass, body weight, plasma insulin, and homeostasis model assessment of insulin resistance. Plasma FGF21 levels were significantly negatively correlated with plasma adiponectin. When subjected to multiple regression analysis, only fat mass was predictive of plasma FGF21 (ß = 0.758; P = 0.004) and CSF FGF21 (ß = 0.767; P = 0.007). The CSF-to-plasma FGF21 ratio was significantly negatively correlated with BMI, fat mass, and plasma FGF21. Subjects in the highest plasma FGF21 quintile had a lower CSF-to-plasma FGF21 ratio (12.7% [9.7-14.9]) compared with those in the lowest plasma FGF21 quintile (94.7% [37.3-99.8]) (P < 0.01).
CONCLUSIONS: Our observations have important implications with respect to the potential central actions of FGF21. Future research should seek to clarify whether FGF21 would be beneficial in the management of obesity and its metabolic complications.
Tan et al., Diabetes. 2011 Sep 16. [Epub ahead of print]
BACKGROUND: Muscle biopsy is the gold standard for diagnosis of mitochondrial disorders because of the lack of sensitive biomarkers in serum. Fibroblast growth factor 21 (FGF-21) is a growth factor with regulatory roles in lipid metabolism and the starvation response, and concentrations are raised in skeletal muscle and serum in mice with mitochondrial respiratory chain deficiencies. We investigated in a retrospective diagnostic study whether FGF-21 could be a biomarker for human mitochondrial disorders.
METHODS: We assessed samples from adults and children with mitochondrial disorders or non-mitochondrial neurological disorders (disease controls) from seven study centres in Europe and the USA, and recruited healthy volunteers (healthy controls), matched for age where possible, from the same centres. We used ELISA to measure FGF-21 concentrations in serum or plasma samples (abnormal values were defined as >200 pg/mL). We compared these concentrations with values for lactate, pyruvate, lactate-to-pyruvate ratio, and creatine kinase in serum or plasma and calculated sensitivity, specificity, and positive and negative predictive values for all biomarkers.
FINDINGS: We analysed serum or plasma from 67 patients (41 adults and 26 children) with mitochondrial disorders, 34 disease controls (22 adults and 12 children), and 74 healthy controls. Mean FGF-21 concentrations in serum were 820 (SD 1151) pg/mL in adult and 1983 (1550) pg/mL in child patients with respiratory chain deficiencies and 76 (58) pg/mL in healthy controls. FGF-21 concentrations were high in patients with mitochondrial disorders affecting skeletal muscle but not in disease controls, including those with dystrophies. In patients with abnormal FGF-21 concentrations in serum, the odds ratio of having a muscle-manifesting mitochondrial disease was 132.0 (95% CI 38.7-450.3). For the identification of muscle-manifesting mitochondrial disease, the sensitivity was 92.3% (95% CI 81.5-97.9%) and specificity was 91.7% (84.8-96.1%). The positive and negative predictive values for FGF-21 were 84.2% (95% CI 72.1-92.5%) and 96.1 (90.4-98.9%). The accuracy of FGF-21 to correctly identify muscle-manifesting respiratory chain disorders was better than that for all conventional biomarkers. The area under the receiver-operating-characteristic curve for FGF-21 was 0.95; by comparison, the values for other biomarkers were 0.83 lactate (p=0.037, 0.83 for pyruvate (p=0.015), 0.72 for the lactate-to-pyruvate ratio (p=0?002), and 0.77 for creatine kinase (p=0.013).
INTERPRETATION: Measurement of FGF-21 concentrations in serum identified primary muscle-manifesting respiratory chain deficiencies in adults and children and might be feasible as a first-line diagnostic test for these disorders to reduce the need for muscle biopsy.
Suomalainen et al.,Lancet Neurol. 2011 Sep;10(9):806-18. Epub 2011 Aug 3.
OBJECTIVE: Fibroblast growth factor (FGF)-21 improves insulin sensitivity and lipid metabolism in obese or diabetic animal models, while human studies revealed increased FGF-21 levels in obesity and type 2 diabetes. Given that FGF-21 has been suggested to be a peroxisome proliferator-activator receptor (PPAR) alpha-dependent regulator of fasting metabolism, we hypothesized that free fatty acids (FFAs), natural agonists of PPARalpha, might modify FGF-21 levels.
RESEARCH DESIGN AND METHODS: The effect of fatty acids on FGF-21 was investigated in vitro in HepG2 cells. Within a randomized controlled trial, the effects of elevated FFAs were studied in 21 healthy subjects (13 women and 8 men). Within a clinical trial including 17 individuals, the effect of insulin was analyzed using an hyperinsulinemic-euglycemic clamp and the effect of PPARgamma activation was studied subsequently in a rosiglitazone treatment trial over 8 weeks.
RESULTS: Oleate and linoleate increased FGF-21 expression and secretion in a PPARalpha-dependent fashion, as demonstrated by small-interfering RNA-induced PPARalpha knockdown, while palmitate had no effect. In vivo, lipid infusion induced an increase of circulating FGF-21 in humans, and a strong correlation between the change in FGF-21 levels and the change in FFAs was observed. An artificial hyperinsulinemia, which was induced to delineate the potential interaction between elevated FFAs and hyperinsulinemia, revealed that hyperinsulinemia also increased FGF-21 levels in vivo, while rosiglitazone treatment had no effect.
CONCLUSIONS: The results presented here offer a mechanism explaining the induction of the metabolic regulator FGF-21 in the fasting situation but also in type 2 diabetes and obesity.
Mai et al. Diabetes. 2009 Jul;58(7):1532-8.
Diabetes mellitus is a major health concern, affecting more than 5% of the population. Here we describe a potential novel therapeutic agent for this disease, FGF-21,
which was discovered to be a potent regulator of glucose uptake in mouse 3T3-L1 and primary human adipocytes. FGF-21–transgenic mice were viable and resistant
to diet-induced obesity. Therapeutic administration of FGF-21 reduced plasma glucose and triglycerides to near normal levels in both ob/ob and db/db mice. These effects persisted for
at least 24 hours following the cessation of FGF-21 administration. Importantly, FGF-21 did not induce mitogenicity, hypoglycemia, or weight gain at any dose
tested in diabetic or healthy animals or when overexpressed in transgenic mice. Thus, we conclude that FGF-21, which we have identified as a novel metabolic
factor, exhibits the therapeutic characteristics necessary for an effective treatment of diabetes.
Kharitonenkov et al. J Clin Invest. 2005 Jun;115(6):1627-35.
(A) H&GE staining of brown fat.
Notice an increase in intensity of brown fat in the FGF-21–transgenic mouse compared with the wild-type mouse. (B) H&GE staining of
subcutaneous white fat. Notice the smaller adipocytes in the FGF-21 mouse compared with the wild type. (C) H&GE staining of livers from
FGF-21–transgenic and wild-type mice. There are no differences between the 2. Magnification, x200 (A–C). (D) PCNA staining of the livers from
FGF-21–infused and saline-treated db/db mice. PNCA immunostaining shows very low proliferation (less than 5%) of hepatocytes (brown staining, arrows) in both the
control and treated groups. Magnification, x400.
|
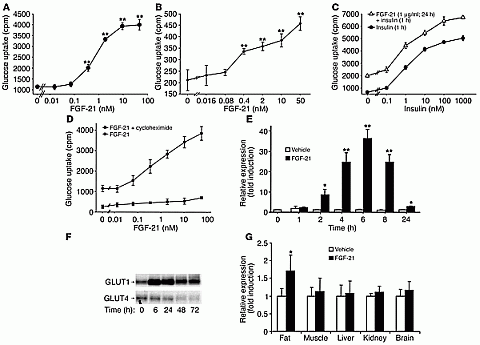 |
The
values (± SE) shown are the average of at least 3 independent measurements. *P
< 0.02, **P < 0.001 compared with no stimulation or vehicle control.
FGF-21 dose response on 3T3-L1 (A) and human primary adipocytes
(B) in glucose uptake
assay. (C) FGF-21
augments insulin activity. Cells were pretreated with or without FGF-21 and then
stimulated with insulin as indicated. (D) Cycloheximide diminishes FGF-21
bioactivity in glucose uptake assay. 3T3-L1 adipocytes were stimulated with
FGF-21 for 24 hours in the presence or absence of cycloheximide. P < 0.001 at
all doses for FGF-21 versus FGF-21 plus cycloheximide stimulations. FGF-21
affects GLUT1 mRNA (E) and protein (F) levels and does not upregulate
GLUT4 protein (F) in 3T3-L1 adipocytes (immunoblot). Cells were starved and then stimulated with
FGF-21 or vehicle as indicated. Quantitative PCR and immunoblotting analyses
were used to measure mRNA and protein levels, respectively. (G) GLUT1 mRNA is upregulated in
adipose tissue of FGF-21–injected ob/ob mice. Two groups (5 animals each) of
8-week-old mice were injected s.c. with FGF-21 or vehicle. Quantitative PCR
analysis was used to measure mRNA |
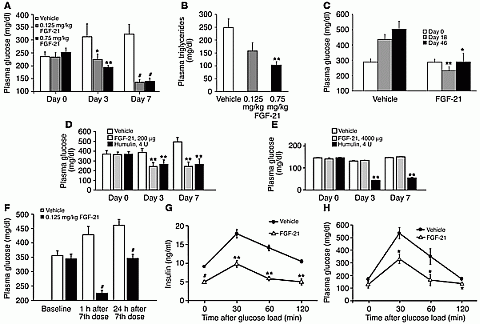 |
The values (± SE) shown are the average
of the measurements of at least 5 animals in a group. *P < 0.05, **P <
0.02, and #P < 0.001 compared with vehicle control. Fed blood glucose
(A) and triglyceride levels (B) in ob/ob
mice treated with FGF-21. FGF-21 was administered once daily, and blood glucose
and triglyceride levels were measured 1 hour after the last injection.
(C) Fed blood glucose levels in db/db mice at days 18 and 46 during 8-week constant-infusion study.
Mice were infused s.c. with 11 µg/kg/h FGF-21 using ALZET minipumps.
(D and E) FGF-21 lowers glucose in obese
ZDF rats and does not induce hypoglycemia in lean ZDF rats. Fed blood glucose
levels were measured in obese (D) and lean (E) ZDF rats that were administered
s.c. twice daily with FGF-21, Humulin, or vehicle at indicated total daily doses
for 1 week. (F) FGF-21 induces extended lowering of fed blood glucose in ob/ob mice. FGF-21 was
administered once daily for 7 days, and blood glucose levels were measured after
the last injection at indicated time points. (G and H) FGF-21 affects insulin levels
(G) and glucose disposal (H) during
OGTT in ob/ob mice. At indicated time points, blood samples were obtained for
glucose and insulin measurements. |
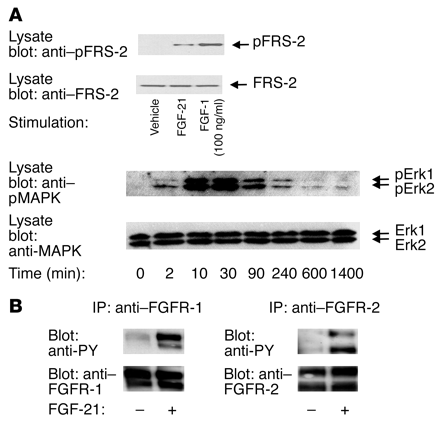 |
(A) FGF-21 induces phosphorylation
of MAPK and FRS-2 in 3T3-L1 adipocytes. Upon stimulation, cells were lysed, and
phospho-specific antibodies were used to determine phosphorylation of MAPK and
FRS-2 in immunoblots. After immunoblots were stripped, anti-MAPK and anti–FRS-2
antibodies were used to confirm that protein loads were equal. For MAPK
experiment, cells were stimulated with FGF-21 for the indicated times. For FRS-2
experiment, cells were stimulated with FGF-21 or FGF-1 (positive control).
(B) FGF-21 stimulates
tyrosine phosphorylation of FGFR-1 and FGFR-2 in 3T3-L1 adipocytes. Cells were
stimulated with FGF-21 and lysed. FGFR-1 and FGFR-2 immunoprecipitates were
analyzed in immunoblots with anti-phosphotyrosine antibodies. After stripping,
anti–FGFR-1 and anti-FGFR-2 antibodies were used to confirm that protein loads
were equal. pErk, phospho-Erk; PY, phosphotyrosine. |
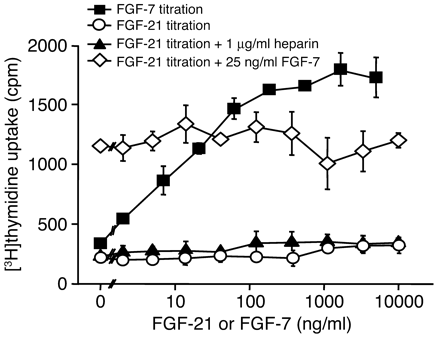 |
FGF-21 does not induce proliferation and does not block FGF-7–dependent
mitogenicity on 4MBr5 cells. Cells were stimulated as indicated with different
concentrations of FGF-7, FGF-21, and FGF-21 in the presence of a constant
concentration of heparin and FGF-21 in the presence of a constant concentration
of FGF-7. |
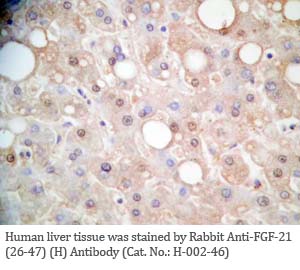 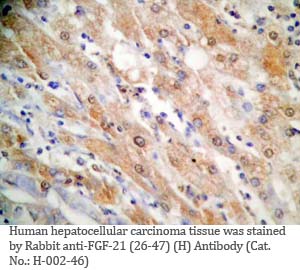
Tissue Sample |
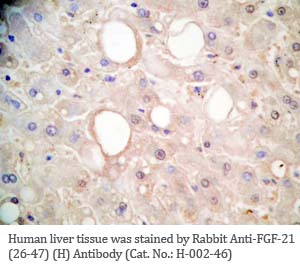
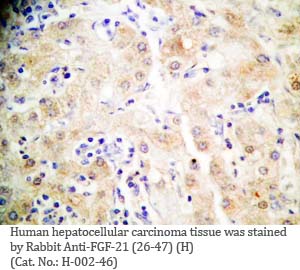
Human liver and hepatocellular sarcinoma tissues |
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody |
Pretreatment |
N/A |
Blocking |
3% H2O2, 2% Normal Goat Serum |
Primary Antibody |
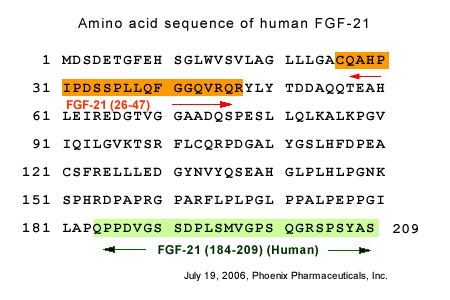
Rabbit anti-FGF-21 (26-47) (Human) antibody (Cat. No.: H-002-46) |
Optimal Dilution |
1:500 |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
Streptavidin-HRP (Vector), 1:400, 30 min |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
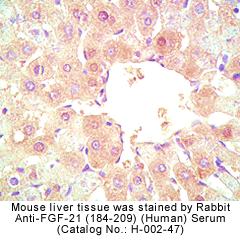 
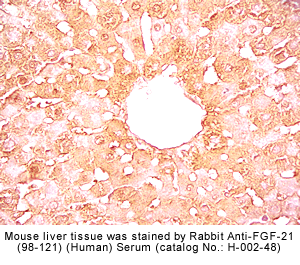
Tissue Sample |
Mouse Liver Tissue |
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody |
Pretreatment |
N/A |
Blocking |
3% H2O2, 2% Normal Goat Serum |
Primary Antibody |
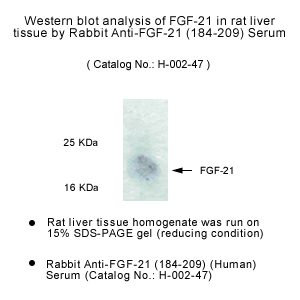
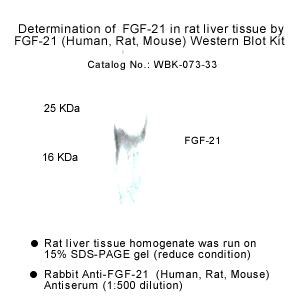
Rabbit anti-FGF-21 (184-209) (Human) antibody (Cat. No.: H-002-47)
Rabbit anti-FGF-21 (26-47) (Human) antibody (Cat. No.: H-002-46) |
Optimal Dilution |
1:500 |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
Streptavidin-HRP (Vector), 1:400, 30 min |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |
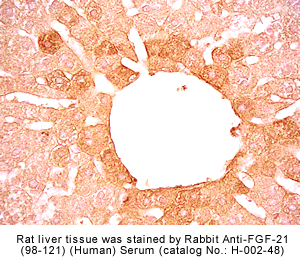 
Fixative |
10% formalin |
Embedding |
Paraffin |
Negative Control |
No primary antibody |
Pretreatment |
N/A |
Blocking |
3% H2O2, 2% Normal Goat Serum |
Primary Antibody |
Rabbit anti-FGF-21 (98-121) (Human) antibody (Cat. No.: H-002-48) |
Optimal Dilution |
1:500 |
Secondary Antibody |
Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification |
Streptavidin-HRP (Vector), 1:400, 30 min |
Detection System |
HRP |
Substrate |
DAB (Sigma), 3 min |
Counterstained |
Hematoxylin, 30 sec |

|
|
|
FGF-19;FGF23;FGF2
%002-47%;%002-46%;%002-48%;%073-33%;%073-32%
|
|
|


