Neuromedin S (NmS)
A Novel anorexigenic hormone and Endogenous Ligand for Orphan GPCR FM-4/TGR-1

We identified a novel 36-amino acid neuropeptide in rat brain as an endogenous ligand for the G protein-coupled receptors FM-3/GPR66 and FM-4/TGR-1, which were identified to date as the neuromedin U (NMU) receptors, and designated this
peptide neuromedin S (NMS) because it was specifically expressed in the suprachiasmatic nucleus (SCN) of the hypothalamus. NMS shared a C-terminal core
structure with NMU. NMS mRNA was highly expressed in the central nervous system, spleen and testis. In rat brain, NMS expression was restricted to the
ventrolateral portion of the SCN and has a diurnal peak under light/dark cycling, but remains stable under constant darkness. Intracerebroventricular (ICV) administration of NMS in rats induced nonphotic type phase shifts in the
circadian rhythm of locomotor activity. ICV injection of NMS also decreased 12-h food intake during the dark period in rats. This anorexigenic effect was more potent than that observed with the same dose of NMU. ICV administration of NMS
increased proopiomelanocortin (POMC) mRNA expression in the arcuate nucleus (Arc)
and corticotropin-releasing hormone mRNA in the paraventricular nucleus, and
induced c-Fos expression in the POMC neurons in the Arc. These findings suggest
that NMS is implicated in the regulation of circadian rhythm and feeding behavior.
Miyazato M, et al. Regul Pept. 2007 Aug 24; [Epub ahead of print].
Neuromedin U (NMU) is a widely spread neuropeptide, with predominant expression
at the gastrointestinal tract and brain, putatively involved in the regulation of
a diversity of biological functions, including food intake, energy balance and
circadian rhythms; all closely related to reproduction. Yet, the implication of
NMU in the control of the gonadotropic axis remains scarcely studied. We report
herein analyses on the hypothalamic expression and function of NMU in different
physiological and experimental states of rat reproductive system. Expression of
NMU mRNA at the hypothalamus was persistently detected along female postnatal
development, with maximum levels in adulthood that fluctuated across the cycle
and were modulated by ovarian steroids. Acute central administration of NMU
evoked increases of serum LH levels in pubertal female rats, while repeated
injection of NMU tended to advance vaginal opening. Likewise, central injection
of NMU increased serum LH concentrations in cyclic female rats, with peak
responses in estrus. In contrast, NMU significantly inhibited pre-elevated LH
secretion in gonadectomized and kisspeptin-treated rats. Finally, in acyclic
females due to photoperiodic manipulation (constant light), hypothalamic NMU mRNA
levels were markedly depressed but relative LH responses to exogenous NMU were
significantly augmented. Altogether, our present data support a predominant
stimulatory role of NMU in the control of the female gonadotropic axis, which
appears under the influence of developmental, hormonal and photoperiodic cues,
and might contribute to the joint regulation of energy balance, biological
rhythms and reproduction. Key words: Neuromedin U (NMU), Luteinizing hormone
(LH), Neuromedin S (NMS), Estrus cycle, Female rat.
Vigo E, et al. Am J Physiol Endocrinol Metab. 2007 Aug 28; [Epub ahead of print]
We recently identified neuromedin S (NMS) as an endogenous ligand for the
FM-4/TGR-1 receptor. Here, we examined the possible involvement of central NMS in
regulation of urinary output and vasopressin (AVP) release in rats.
Intracerebroventricular (icv) injection of NMS induced a dose-dependent increase
in the plasma level of AVP, followed by a decrease of nocturnal urinary output.
Expression of cFos after icv injection of NMS was observed in the supachiasmatic
nucleus (SCN), arcuate nucleus, paraventricular nucleus (PVN), and supraoptic
nucleus (SON). The cFos expressing cells in PVN and SON, but not SCN, were then
double-stained using antibodies against the vasopressin. On the other hand, icv
injection of neuromedin U, which also binds to the FM-4/TGR-1 receptor, required
a concentration ten times higher than that of NMS in order to exert the same
antidiuretic potency. These results suggest that central NMS may exert a
physiological antidiuretic action via vasopressin release.
Sakamoto T, et al. Biochem Biophys Res Commun. 2007 Sep 21;361(2):457-61. Epub 2007 Jul 18.
Neuromedin S (NMS) and neuromedin U (NMU) are regulatory peptides that share the
C-terminal amino-acid sequence and act via common G protein-coupled receptors
called NMUR1 and NMUR2. Semiquantitative real time-PCR showed that in the rat
hypothalamus and testis NMS gene expression was markedly higher than that of the
NMU gene, while the reverse occurred in the anterior pituitary and thyroid gland.
Low expression of both genes was detected in the thymus, adrenal gland and ovary,
whereas in the pancreatic islets only the expression of NMU mRNA was detected. In
the rat hypothalamus the expression of the NMUR2 gene was strikingly higher than
that of the NMUR1 gene; in contrast, in the testis and ovary the very low
expression of NMUR2 contrasted with the relatively high expression of the NMUR1
gene. In the other glands examined only expression of the NMUR1 gene was found.
The marked differences in the level of expression of NMU, NMS and their receptors
in the hypothalamus and endocrine glands of the rat suggest that in this species
such neuromedins may play different roles in the functional regulation of
neuroendocrine axes.
Rucinski M, et al. Int J Mol Med. 2007 Aug;20(2):255-9.
Neuromedin S (NMS), a 36 amino acid peptide structurally related to neuromedin U, was recently identified in rat brain as ligand for the G protein-coupled receptor FM4/TGR-1, also termed neuromedin U receptor type-2 (NMU2R). Central expression
of NMS appears restricted to the suprachiasmatic nucleus, and NMS has been involved in the regulation of dark-light rhythms and suppression of food intake.
Reproduction is known to be tightly regulated by metabolic and photoperiodic cues. Yet the potential contribution of NMS to the control of reproductive axis
remains unexplored. We report herein analyses of hypothalamic expression of NMS and NMU2R genes, as well as LH responses to NMS, in different developmental and
functional states of the female rat. Expression of NMS and NMU2R genes was detected at the hypothalamus along postnatal development, with significant
adulthood). In adult females, hypothalamic expression of NMS (which was confined to suprachiasmatic nucleus) and NMU2R significantly varied during the estrous
cycle (maximum at proestrus) and was lowered after ovariectomy and enhanced after progesterone supplementation. Central administration of NMS evoked modest LH
secretory responses in pubertal and cyclic females at diestrus, whereas exaggerated LH secretory bursts were elicited by NMS at estrus and after
short-term fasting. Conversely, NMS significantly decreased elevated LH concentrations of ovariectomized rats. In summary, we provide herein novel
evidence for the ability of NMS to modulate LH secretion in the female rat. Moreover, hypothalamic expression of NMS and NMU2R genes appeared dependent on
the functional state of the female reproductive axis. Our data are the first to
disclose the potential implication of NMS in the regulation of gonadotropic axis, a function that may contribute to the integration of circadian rhythms, energy balance, and reproduction.
Vigo E, et al. Endocrinology. 2007 Feb;148(2):813-23. Epub 2006 Nov 16.


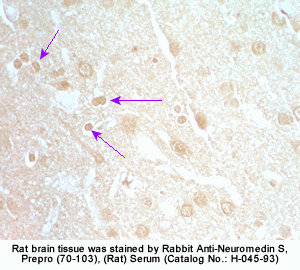
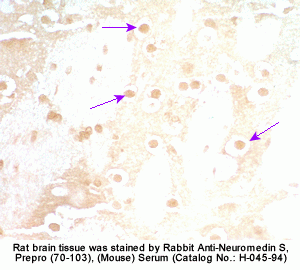
The discovery of neuropeptides has resulted in an
increased understanding of novel regulatory mechanisms of certain
physiological phenomena. Here we identify a novel neuropeptide of 36
amino-acid residues in rat brain as an endogenous ligand for the
orphan G protein-coupled receptor FM-4/TGR-1, which was identified
to date as the neuromedin U (NMU) receptor, and designate this
peptide 'neuromedin S (NMS)' because it is specifically expressed in
the suprachiasmatic nuclei (SCN) of the hypothalamus. NMS shares a
C-terminal core structure with NMU. The NMS precursor contains
another novel peptide. NMS mRNA is highly expressed in the central
nervous system, spleen and testis. In rat brain, NMS expression is
restricted to the core of the SCN and has a diurnal peak under
light/dark cycling, but remains stable under constant darkness.
Intracerebroventricular administration of NMS in rats activates SCN
neurons and induces nonphotic type phase shifts in the circadian
rhythm of locomotor activity. These findings suggest that NMS in the
SCN is implicated in the regulation of circadian rhythms through
autocrine and/or paracrine actions.
Mori K, et al. EMBO J. 2005 Jan 06; [Epub ahead of print]
A novel 36-amino acid neuropeptide, neuromedin S
(NMS), has recently been identified in rat brain and has been shown
to be an endogenous ligand for two orphan G protein-coupled
receptors, FM-3/GPR66 and FM-4/TGR-1. These receptors have been
identified as neuromedin U (NMU) receptor type-1 and type-2,
respectively. In this study, the physiological role of the novel
peptide, NMS, on feeding regulation was investigated.
Intracerebroventricular (icv) injection of NMS decreased 12-h food
intake during the dark period in rats. This anorexigenic effect was
more potent and persistent than that observed with the same dose of
NMU. Neuropeptide Y, ghrelin and agouti-related protein-induced food
intake was counteracted by co-administration of NMS. Icv
administration of NMS increased proopiomelanocortin (POMC) mRNA
expression in the arcuate nucleus (Arc) and CRH (CRH) mRNA in the
paraventricular nucleus (PVN). Pretreatment with SHU9119 (antagonist
for -melanocyte-stimulating hormone) and -helical CRF- (9-41)
(antagonist for CRH) attenuated NMS-induced suppression of 24 h food
intake. After icv injection of NMS, Fos-immunoreactive cells were
detected in both the PVN and Arc. When neuronal multiple unit
activity was recorded in the PVN before and after icv injection of
NMS, a significant increase in firing rate was observed 5 min after
administration, and this increase continued for 100 min. These
results suggest that the novel peptide, NMS, may be a potent
anorexigenic hormone in the hypothalamus, and that expression of
POMC mRNA in the Arc and CRH mRNA in the PVN may be involved in NMS
action on feeding.
Ida T., et al. Endocrinology 2005, June 23, 10.1210/en.2005-0107
 |
 |
 |
| |
| NMU receptor type-1 (NMU1R) |
NMU receptor type-2 (NMU2R) |
| FM-3/GPR66 |
FM-4/TGR-1 |
| Peripheral tissues |
CNS: PVN & SCN |
| NMU = NMS |
NMS > NMU |
|
|
 |
 |
 |
 |
 |
 |
| |
| Neuromedin U |
Neuromedin S |
| Brain-gut neuropeptide |
Neuro & Immunopeptide |
| Suppression of feeding |
Circadian rhythm regulation in the SCN |
| Regulation of energy homeostasis |
Immune response in the spleen |
| Elevation of arterial blood pressures and control of local blood flow |
Spermatogenesis in the testis |
|
|
 |
 |
 |

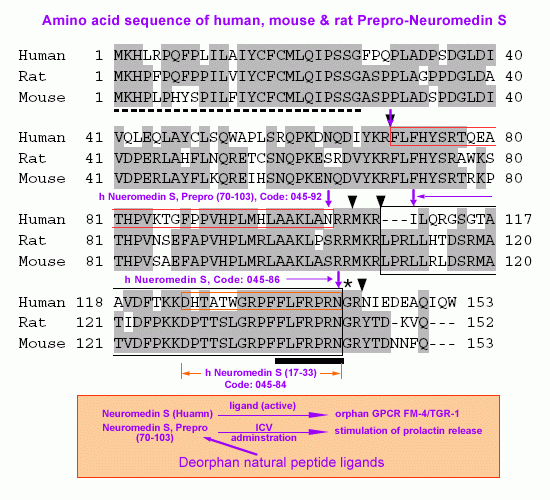
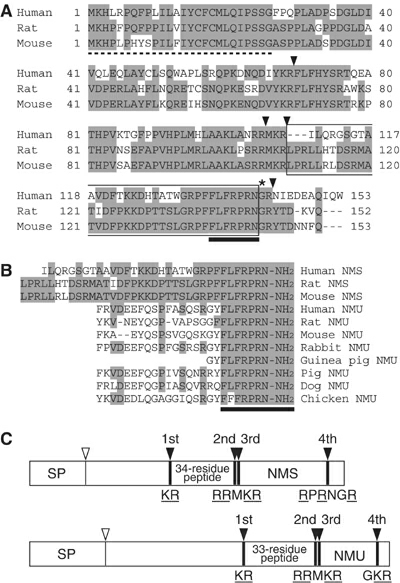
Structure of NMS. (A) Amino-acid sequences of
human, rat and mouse prepro-NMS. Identical residues are shaded. The
dotted line denotes the predicted signal peptide. The arrowheads
indicate proteolytic processing sites. The asterisk shows a glycine
residue, which serves as an amide donor for C-terminal amidation.
NMS sequences are boxed. Sequences conserved between NMS and NMU are
indicated by a solid underline. The sequence data for the human, rat
and mouse NMS cDNAs have been submitted to the DDBJ/EMBL/GenBank
databases under accession nos. AB164464, AB164465 and AB164466,
respectively. (B) Sequence comparison of NMS and NMU. Human, rat and
mouse NMS and NMU sequences are aligned. Residues identical between
peptides are shaded. Conserved core sequences are indicated by a
solid underline. (C) Schematic representation of the preproproteins
of rat NMS and NMU. The preproproteins of NMS and NMU are
represented by boxes divided into protein domain, proportional to
their length. The open and filled arrowheads indicate cleavage sites
by signal peptidase and proprotein convertase, respectively. The
sequences of the proteolytic processing sites are shown. The basic
amino-acid residues recognized by proprotein convertase are
underlined. SP, signal peptide.
Mori K, et al. EMBO J. 2005 Jan 06; [Epub ahead of print]
Effects of NMS on rat systemic blood pressure
compared with the effects of NMU. (C, D) Rat systemic blood
pressure. The indicated amounts of NMS (C) and NMU (D) were
administered to rats at the time indicated by the arrow. Both assays
were performed as described in "Supplementary materials and
methods."
Mori K, et al. EMBO J. 2005 Jan 06; [Epub ahead of print]
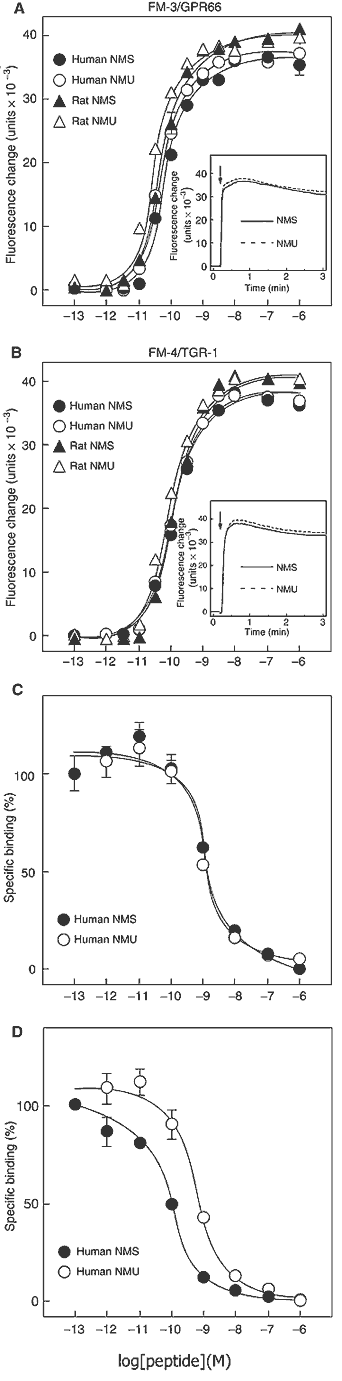

(A, B) Dose-response relationships of [Ca2+]i change for
human NMS (filled circle), human NMU (open circle), rat NMS (filled
triangle) and rat NMU (open triangle) in CHO/FM-3 (A) and CHO/FM-4
(B) cells. Data points are meanss.e.m. of triplicates for each
experiment. Insets show the time course of [Ca2+]i changes induced
by human NMS (solid line) and human NMU (dotted line). Each peptide
(10-8 M) was added at the time indicated by the arrow. (C, D)
Competitive radioligand binding analysis. [125I-Tyr0]-human NMS
binding to FM-3/GPR66 (C) and FM-4/TGR-1 (D) was displaced by
increasing concentrations of human NMS (filled circle) and human NMU
(open circle). Data were determined in triplicate.
Mori K, et al. EMBO J. 2005 Jan 06; [Epub ahead of print]
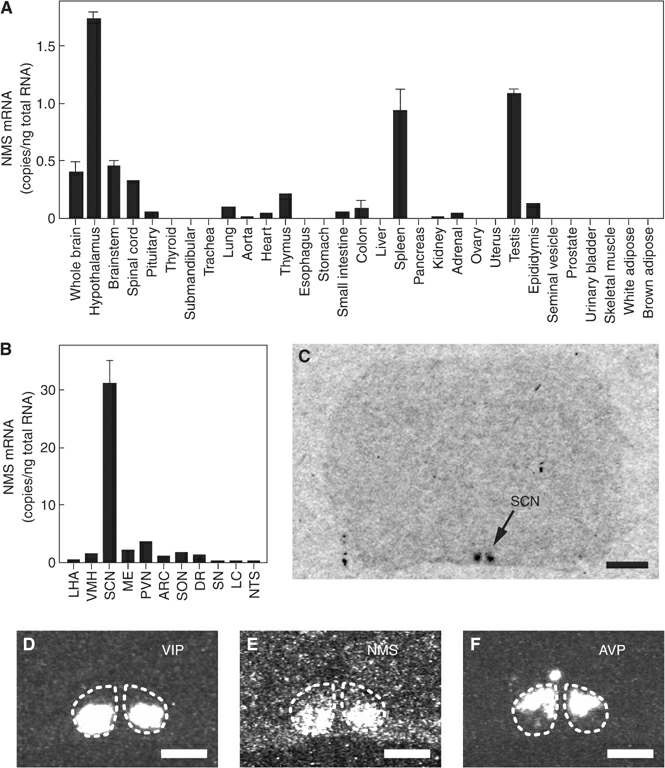
(A, B) Quantitative RT-PCR
analysis of the NMS mRNA in a rat multiple-tissue cDNA panel. Each
column represents the means.e.m. of triplicate experiments. LHA,
lateral hypothalamic area; VMH, ventromedial hypothalamus; SCN,
suprachiasmatic nucleus; ME, median eminence; PVN, paraventricular
nucleus; ARC, arcuate nucleus; SON, supraoptic nucleus; DR, dorsal
raphe; SN, substantia nigra; LC, locus coeruleus; NTS, nucleus of
the solitary tract. (C) Autoradiogram of the NMS mRNA expression in
a coronal section of the rat brain. Scale bar, 2 mm. (D-F)
Distribution of VIP (D), NMS (E) and AVP (F) mRNA in the rat SCN.
Serial sections were used. The SCN is indicated with a dotted line.
Scale bar, 500 m.
Mori K, et al. EMBO J. 2005 Jan 06; [Epub ahead of print]
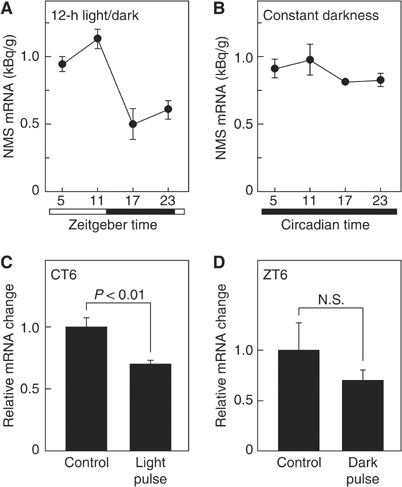

(A, B) Temporal expression profiles of the NMS mRNA. Animals were
maintained under 12-h light/dark cycles (A) or constant darkness for
2 days (B). The amount of NMS mRNA was quantified by in situ
hybridization analysis. The experiments were performed side by side.
Data represent the meanss.e.m. of 3-4 animals. Open and filled
horizontal bars indicate light and dark periods, respectively. (C,
D) Responses of NMS expression to light exposure under conditions of
constant darkness and to a dark pulse during the light period of a
12-h light/dark cycle. Animals maintained in constant darkness for 2
days were exposed to a light pulse for 30 min at CT6 (C), or animals
maintained under 12-h light/dark cycles were exposed to a dark pulse
for 30 min at ZT6 (D). Brain samples were collected 1 h after
exposure to the light or dark pulse. Data are presented as the
meanss.e.m. of 3-4 animals, and are shown as relative changes in NMS
mRNA levels.
Mori K, et al. EMBO J. 2005 Jan 06; [Epub ahead of print]
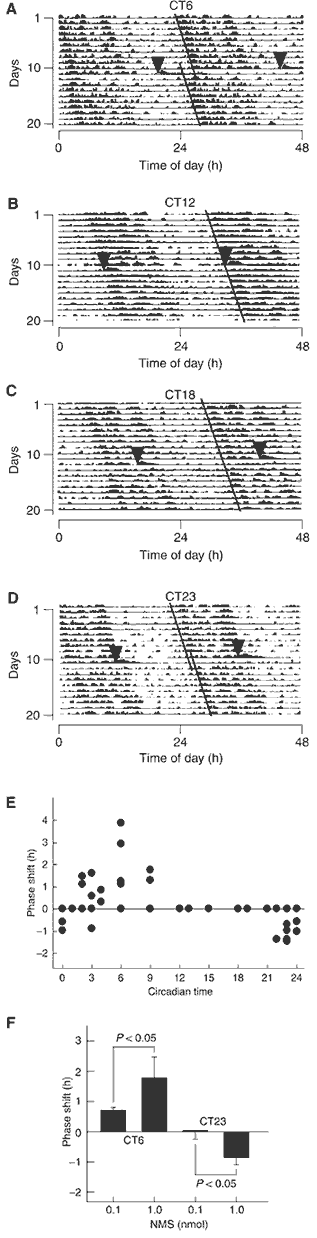
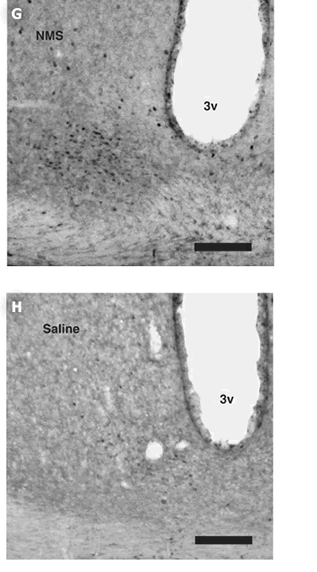

(A-D) Phase shifts of the
circadian rhythm of locomotor activity induced by ICV administration
of NMS. Representative doubled-plotted actograms of locomotor
activity are shown. Rats were ICV administered with rat NMS (1 nmol)
at CT6 (A), CT12 (B), CT18 (C) and CT23 (D). Each arrowhead
indicates the time of NMS administration. Each line shows the
regression lines drawn based on the daily onset of locomotor
activity before and after administration. (E) Phase-response plot
for ICV administration of 1 nmol NMS. The plus and minus values
indicate phase advance and delay, respectively. n=3-5. The data
points at CT12, 13, 15, 18 and 19 overlap. (F) Dependence of
circadian rhythm phase shift on NMS administration. ICV
administration of NMS at CT6 and CT23 induced significant phase
advance and delay, respectively, in a dose-dependent manner. n=4-5
per group. (G, H) Induction of c-Fos protein expression within the
SCN following ICV administration of NMS. Rats were administered at
CT6 with 1 nmol NMS (G) or saline (H). 3v, third ventricle. Scale
bar, 150 m.
Mori K, et al. EMBO J. 2005 Jan 06; [Epub ahead of print]
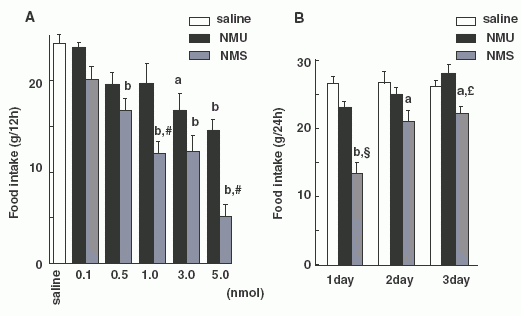
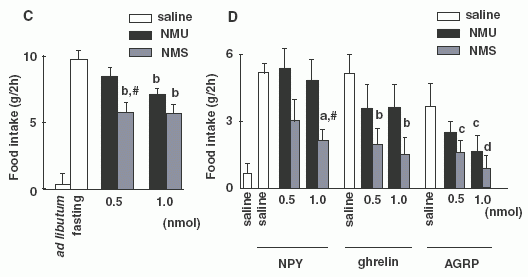
Each
bar and vertical line represents the mean ± SEM. (A) Dark-phase
feeding. Food intake of free-feeding rats was examined during a
period of 12 h from 1900 h to 0700 h. Each reagent was injected at
1845 h (a, P < 0.05; b, P < 0.01 vs. saline; #, P < 0.01
vs. NMU at the same dose as NMS). (B) The inhibitory effects on food
intake after injection. Food intake was examined over a period of 24
h from 1900 h to 1900 h for 3 days after injection. Each reagent (1
nmol) was injected at 1845 h. (a, P < 0.05; b, P < 0.01 vs.
saline; £, P < 0.05; §, P < 0.01 vs. NMU at the same dose as
NMS). (C) Two-hour food intake in rats that had fasted for 8 h and
then received each reagent at 0900 h (b, P < 0.01 vs. saline in
fasting rats; #, P < 0.01 vs. NMU at same dose as NMS in fasting
rats). (D) Effect of co-administration of NPY (0.5 nmol), ghrelin
(0.5 nmol) or AGRP (1 nmol) with NMU or NMS on 2-h food intake in
free-feeding rats. Each reagent was injected at 0845 h (a, P <
0.05 vs. NPY + saline; #, P < 0.05 vs. NPY + 1.0 nmol NMU; b, P
< 0.01 vs. ghrelin + saline; c, P < 0.05; d, P < 0.01 vs.
AGRP + saline).
Ida T., et al. Endocrinology 2005, June 23, 10.1210/en.2005-0107
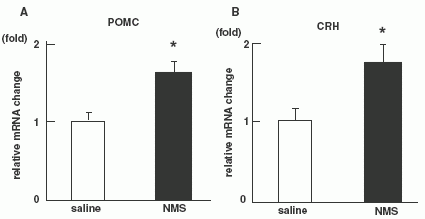

Quantitative RT-PCR on NMS administered rats (n = 8 per
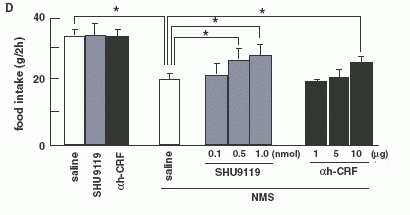
group). Level of POMC mRNA (A) and CRH mRNA (B). (C):Effect of
pre-treatments with a-MSH antagonist (SHU9119) or CRH antagonist
(a-helical CRF-(9-41)) on food intake reduction by NMS in 8-h fasted
rats. Each antagonist was injected at 0745 h, and then NMS was
injected at 0845 h. Food intake was examined during a 2-h period
from 0900 h to 1100 h. Asterisks indicate the significant difference
(P < 0.05). (D): Effect of pre-treatments with a-MSH antagonist
(SHU9119) or CRH antagonist (a-helical CRF-(9-41)) on food intake
reduction by NMS in intact rats. Each antagonist was injected at
0745 h, and then NMS was injected at 0845 h, then, 24 h food intake
was examined. Asterisks indicate the significant difference (P <
0.05).
Ida T., et al. Endocrinology 2005, June 23, 10.1210/en.2005-0107


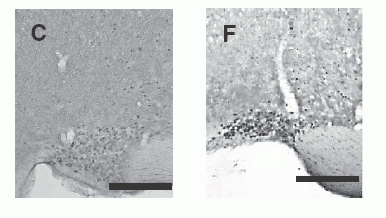
c-Fos-immunoreactive cell
nuclei in selected brain regions after administration of 1 nmol NMS
(A, B, C) or NMU (D, E, F). The brain regions shown include the PVN
(A, D), Arc (B, E) and SON (C, F). Scale bar = 200 µm (for all
panels).
Ida T., et al. Endocrinology 2005, June 23, 10.1210/en.2005-0107

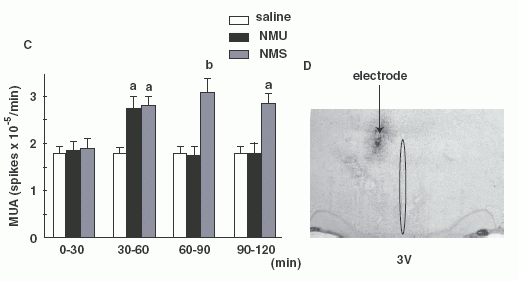
(A, B): Frequency-time histograms of
firing rate of the PVN after administration of NMS or NMU in
representative rats. NMS (A) or NMU (B) was injected 15 min (arrows)
after stable MUA volley. Abscissa: time (min), ordinate: spike/sec.
MUA profiles in one representative rat are shown. (C): Summary of
the effects of icv injection of NMS or NMU on MUA volley frequency
at 30 min intervals for 2-h. Each column and vertical bar represents
the mean ± SEM (n = 3-4) (a, P < 0.05 vs. saline; b, P < 0.01
vs. saline). (D):Location of the MUA electrode tip. Recording sites
of MUA volleys were located adjacent to the PVN.
Ida T., et al. Endocrinology 2005, June 23, 10.1210/en.2005-0107


