- The first endogenous ligand for receptor FPRL2
- Binding to formylated peptide receptor (FPR).
- Inhibiting antigen-derived cellular proliferation and cytokine production.
- Enabling anti-migratory action.
Protecting against experimental myocardial ischemia-reperfusion.
The anti-inflammatory properties of annexin-1 peptides have been largely ascribed
to their powerful antineutrophil actions in vivo. We have recently reported
that the N-terminal fragment of annexin-1, Anx-1(2-26), preserves contractile
function of cardiac muscle in vitro. The aim of the present study was to
determine if Anx-1(2-26) elicits protective actions specifically on the cardiac
myocyte (in the absence of neutrophils), using a model of metabolic inhibition
to simulate ischaemia.Metabolic inhibition of cardiac myocytes (4 h incubation
at 37 degrees C in HEPES-containing buffer supplemented with 2-deoxy-D-glucose,
D,L-lactic acid and pH adjusted to 6.5) followed by 2.5 h recovery in normal
medium markedly increased creatine kinase (CK) and lactate dehydrogenase (LDH)
levels by 179+/-39 and 26+/-7 IU L(-1) (both n=40, P<0.001), respectively.
However, cellular injury was significantly decreased when Anx-1(2-26) (0.3 muM)
was present during metabolic inhibition, CK by 74+/-10% and LDH by 71+/-6%
(both n=31, P<0.001), respectively.Boc 2 (10 muM), a nonselective formyl
peptide receptor antagonist, present during metabolic inhibition, abolished the
cardioprotective effect of Anx-1(2-26).Addition of chelerythrine (10 muM),
5-hydroxydecanoate (500 muM) or SB202190 (1 muM) during metabolic inhibition
also abolished Anx-1(2-26)-induced cardioprotection.Cellular injury induced by
metabolic inhibition was also largely prevented when myocytes were incubated
with Anx-1(2-26) for 5 min with 10 min recovery prior to the insult, or when
Anx-1(2-26) was present only during the recovery period following drug-free
metabolic inhibition.In conclusion, the annexin-1 peptide Anx-1(2-26) potently
prevents cardiac myocyte injury induced by metabolic inhibition, an action that
was dependent at least in part on the activation of the formyl peptide receptor
family of G-protein-coupled receptors, protein kinase C, p38 mitogen-activated
protein kinase and ATP-sensitive potassium channels.
British Journal of Pharmacology advance online publication, 11 April 2005; doi:10.1038/sj.bjp.0706211.
Ritchie RH, et al. Br J Pharmacol. 2005 Apr 11; [Epub ahead of print]
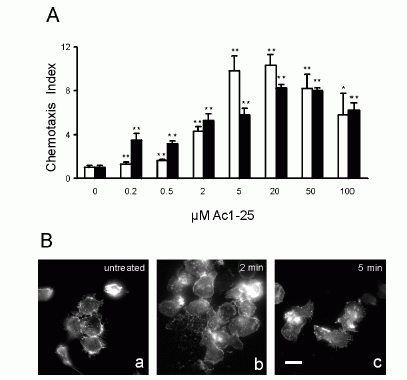
FIGURE 1. Directional migration of human peripheral blood granulocytes and
monocytes. A, Chemotactic activity of the annexin 1 peptide Ac1-25 for human
granulocytes and monocytes. Migration of granulocytes () or monocytes (f) in
response to different peptide concentrations is presented as the average
chemotaxis indexSEM of triplicate wells. , p�0.05, , p � 0.01. B, The
annexin 1 peptide Ac1-25 alters neutrophil morphology and F-actin distribution.
Rhodamine-phalloidin staining of F-actin in unstimulated granulocytes (a) or
cells treated with 50 M Ac1-25 for 2 min (b) or 5 min (c). Cells were fixed
following treatment and analyzed by fluorescence microscopy. Note that Ac1-25
causes remodeling of the actin cytoskeleton, cell polarization, and spreading.
Bar represents 10 m. .01. Ernst S, et al. An annexin 1 N-terminal Peptide
activates leukocytes by triggering different members of the formyl Peptide
receptor family.
J Immunol. 2004 Jun 15;172(12):7669-76
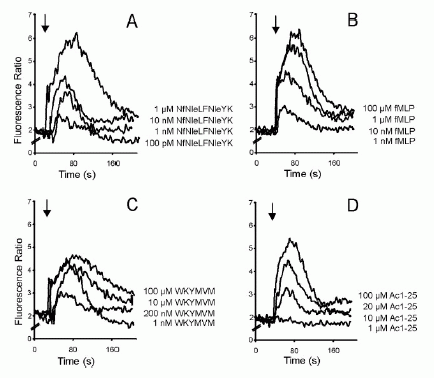
FIGURE 2. Dose-response curves of intracellular Ca2 mobilization in
monocytes. Monocytes loaded with fura 2-AM were stimulated with increasing
concentrations of the fMLP analog NfNleLFNleYK (A), fMLP (B), WKYMVM (C) or
Ac1-25 (D). Fluorescence ratios of representative recordings are presented.
Arrows indicate agonist addition. .01. Ernst S, et al. An annexin 1 N-terminal
Peptide activates leukocytes by triggering different members of the formyl
Peptide receptor family.
J Immunol. 2004 Jun 15;172(12):7669-76
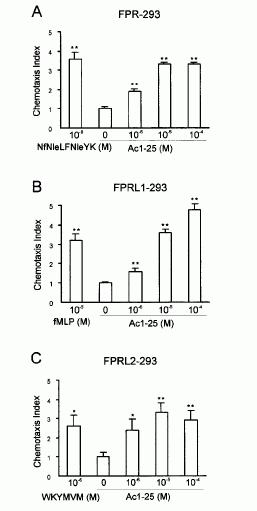
FIGURE 3. Annexin 1 can use all members of the FPR family to induce
chemotaxis. Various concentrations of chemoattractant were added in the lower
wells of a chemotaxis chamber. HEK 293 cells stably expressing FPR, FPRL1, or
FPRL2, respectively (106 cells/ml), were placed in the upper wells. Following
incubation the number of cells that had migrated toward the chemoattractant
source was determined as described in Materials and Methods. Cell migration is
expressed as the average chemotaxis index (mean SEM) of six wells. A,
Migration of FPR-293 cells in response to Ac1-25 or the f MLP analog NfNleLFNleYK is
shown. B, The migration of FPRL1-293 cells in response to Ac1-25 or f MLP and (C) migration of
FPRL2-293 cells in response to Ac1-25 or the synthetic peptide WKYMVM. , p �
0.05, , p � 0 .01. Ernst S, et al. An annexin 1 N-terminal Peptide activates
leukocytes by triggering different members of the formyl Peptide receptor
family.
J Immunol. 2004 Jun 15;172(12):7669-76
References:
1. Kamal A.M., et al. An annexin 1 (ANXA-1)-derived peptide inhibits prototype antigen-deriven human T
cell Th1 and Th2 responses in vitro. Clin. Exp. Allergy 31, 1116-1125 (2001)
2. Perretti M., et al. Involvement of the receptor for formylated peptides in the in vivo
anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 158, 1969-1973 (2001)
3. La M, et al. Annexin 1 peptides protect against experimental myocardial
ischemia-reperfusion: analysis of their mechanism of action. FASEB J. 15, 2247-2256 (2001)
4. Ernst S, et al. An annexin 1 N-terminal Peptide activates leukocytes by triggering different
members of the formyl Peptide receptor family. J Immunol. 2004 Jun 15;172(12):7669-76
We have previously shown that the glucocorticoid dexamethasone prevents the
cardiodepressant actions of interferon-gamma plus lipopolysaccharide in cardiac
tissue in vitro. We now demonstrate that an N-terminal fragment of annexin-1
(Ac2-26, 1 microM), a putative mediator of glucocorticoid actions, completely
protects against interferon-gamma+lipopolysaccharide-induced depression of the
inotropic response to isoprenaline in rat isolated papillary muscles. However,
Ac2-26 does not preserve resting contractile function. Fifteen hours incubation
with interferon-gamma+lipopolysaccharide also markedly induced mRNA expression
(by real time polymerase chain reaction, PCR) of both the nitric oxide synthase
2 (NOS2) isoform of nitric oxide synthase (by 6.7 +/- 1.7-fold, P < 0.01)
and cyclo-oxygenase-2 (by 3.4 +/- 0.6-fold, P < 0.05) in cardiomyocytes.
Pretreatment with Ac2-26 (1 microM) prevented the induction of
cyclo-oxygenase-2 mRNA, but not NOS2 mRNA, whereas dexamethasone (1 microM)
suppressed the expression of both NOS2 mRNA and cyclo-oxygenase-2 mRNA.
Co-incubation of dexamethasone with an anti-annexin-1 antibody did not
attenuate the suppression of NOS2 mRNA. Thus, Ac2-26 reproduces some, but not
all, of the cardioprotective effects of glucocorticoids in vitro in the absence
of neutrophils. These protective actions are independent of changes in NOS2
expression.
Ritchie RH, Sun X, Bilszta JL, Gulluyan LM, Dusting GJ. Eur J Pharmacol 2003 Feb 14;461(2-3):171-9
Myocardial reperfusion injury is associated with the infiltration of
blood-borne polymorphonuclear leukocytes. We have previous described the
protection afforded by annexin 1 (ANXA1) in an experimental model of rat
myocardial ischemia-reperfusion (IR) injury. We examined the 1) amino acid
region of ANXA1 that retained the protective effect in a model of rat heart IR;
2) changes in endogenous ANXA1 in relation to the IR induced
damage and after pharmacological modulation; and 3) potential involvement of
the formyl peptide receptor (FPR) in the protective action displayed by ANXA1
peptides. Administration of peptide Ac2-26 at 0, 30, and 60 min postreperfusion
produced a significant protection against IR injury, and this was associated
with reduced myeloperoxidase activity and IL-1beta levels in the infarcted
heart. Western blotting and electron microscopy analyses showed that IR heart
had increased ANXA1 expression in the injured tissue, associated mainly with the infiltrated leukocytes. Finally, an
antagonist to the FPR receptor selectively inhibited the protective action of
peptide ANXA1 and its derived peptides against IR injury. Altogether, these
data provide further insight into the protective effect of ANXA1 and its
mimetics and a rationale for a clinical use for drugs developed from this line
of research.
La M, et al. FASEB J 2001 Oct;15(12):2247-56
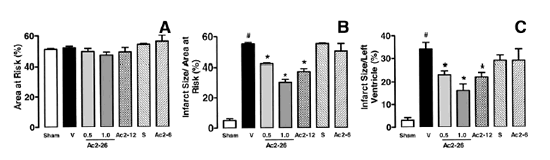
Effects of ANXA1-derived peptides on myocardial ischemia-reperfusion injury. Rats were
treated i.v. with saline (1 ml/kg), peptide Ac2?6 (0.5 and 1 mg/kg), peptide
Ac2?2 (1 mg/kg), scramble Ac2?2 (S, 1 mg/kg), or peptide Ac2? (1 mg/kg) at
the end of the 25 min ischemic period. Tissues were analyzed 2 h after
reperfusion; the area at risk (A), infarct size/area at risk (B),
infarct size/left ventricle (C) were determined as described in
Materials and Methods. A group of sham-operated animals (sham) was also
evaluated. Data are means ?SE of n = 5?4 rats per group. #P < 0.05 vs. sham
and *P < 0.05 vs. saline treatment.
La M, et al. FASEB J 2001 Oct;15(12):2247-56
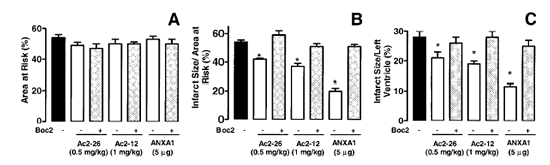
Boc2 reversed the cardioprotective actions of ANXA1 and its NH2 terminus
peptides. The effect of peptide Ac2?6 (0.5 mg/kg) and ANXA1 (25 µg/kg)
administered alone or together with 0.4 mg/kg Boc2. Drugs were administered 25
min after ischemia and tissues were analyzed 2 h after reperfusion; the area at
risk (A), infarct size/area at risk (B), and infarct size/left
ventricle (C) were determined as described in Materials and Methods.
Data are means ?SE of n = 5 rats per group. *P < 0.05 vs. saline
treatment.
La M, et al. FASEB J 2001 Oct;15(12):2247-56

Expression of endogenous ANXA1 in myocardial samples. A) Western blot analysis of
endogenous ANXA1 expression in sham (S), naive (N) animals, and those subjected
to IR treated with either saline (C) or 1 mg/kg peptide Ac2?6 (P). B)
Densitometric analysis for ANXA1 37 and 34 kDa isoforms. Values are mean ?SE of n = 5? rats
per group. *P < 0.01 vs. sham treatment; #P < 0.01 vs.
saline group.
La M, et al. FASEB J 2001 Oct;15(12):2247-56
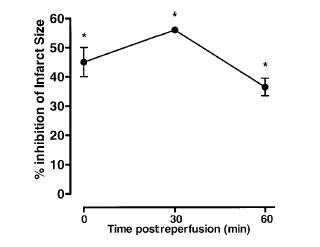
Time course of peptide Ac2?6 cardioprotective effect. Rats were given peptide
Ac2?6 (1 mg/kg i.v.) immediately after the start of reperfusion (time 0), 30,
and 60 min after reperfusion, and tissue was collected at 120 min to determine
the infarct size/area at risk value as described in Materials and Methods.
Infarct size was significantly reduced at all times vs. control (IS/AR
value=55?%, n=6). Data are mean ?SE of n = 4? rats per group. *P < 0.05 vs. control.
La M, et al. FASEB J 2001 Oct;15(12):2247-56
A 4 °C equilibrium ligand binding assay, was used to determine the number of N-formyl peptide receptors on the plasma membrane surface.
N-formyl-norleucyl-leucyl-phenylalanyl-norleucyl-tyrosyl-lysine-fluorescein
(FNLPNTL-FL) stocks were made in dimethyl sulfoxide (Me2SO) and were
diluted to a final concentration of less than 0.01% Me2SO for each
assay.
Samples were equilibrated for 1 h at 4 °C in the dark, then a flow
cytometer (FACScan, Becton-Dickinson), calibrated with fluorescein
isothiocyanate-labeled beads (Quantum 24, Flow Cytometry Standards, Research
Triangle Park, NC), was used to quantify fluorescence binding per cell.
Neutrophils were gated based on forward and side scatter parameters.
Nonspecific binding was determined in the presence of 3 ?nbsp;10-5
M F-Met-Leu-Phe (FMLP) and subtracted from total binding to give
specific binding. Two lots of calibration beads were utilized during the course
of this study. When the second lot was received, it was checked against the
first lot, and both sets gave the same slope for fluorescence mean channel number
versus fluorescein equivalents listed by the manufacturer. Thus the
standards were internally consistent and stable over the course of the
experiments.
In addition, one fluorescent bead standard from one lot was calibrated by comparison with known concentrations
of fluorescein and found to be within 6% of the value reported by the
manufacturer. Sensitivity analysis showed that this small a difference in
determination of Rs had no effect on calculation of the binding rate
constants, and the nominal values for fluorescein equivalents per bead provided
by the manufacturer were used for converting mean channel number to fluorescein
equivalents per cell. Data for specific binding in fluorescein equivalents were
converted to FNLPNTL-FL number per cell using a conversion factor of
1.22 FNLPNTL-FL equivalents/fluorescein equivalent. Nonlinear regression
of FNLPNTL-FL bound/cell versus free ligand using the equilibrium
solution of Reaction I yielded the total number of receptors on the cell
surface (Rs) and the equilibrium dissociation constant (Kd).
We note that for a two-state interconverting receptor model, an equilibrium
binding curve may yield a Kd that is intermediate to the Kd
values of the individual species, but the maximum number of receptors will be
unchanged.
Julie F. Hoffman and Jennifer J. Linderman. Receptor Up-regulation, Internalization, and Interconverting Receptor States ---CRITICAL COMPONENTS
OF A QUANTITATIVE DESCRIPTION OF N-FORMYL PEPTIDE-RECEPTOR DYNAMICS IN THE NEUTROPHIL. JBC,Volume 271, Number 31, Issue of August 2, 1996 pp. 18394-18404


