|
|
|
LL37 (Human), KR-12 & WLBU2 |
|
A
Cathelicidin-derived
Peptide with antimicrobial and angiogenic activity |
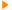 More
peptide ligands for Formylated Peptide Receptor More
peptide ligands for Formylated Peptide Receptor
|
A wall of the urinary tract surrounding the lumen is depicted. Bacteria ascend against the flow of urine. A thin film of fluid covers the lumenal surface of the epithelium and represents the 'battlefield' where microbe and cathelicidins meet. From the bottom to the top of the figure:
(1) cathelicidin is constitutively expressed in the absence of bacteria;
(2) microbes ascend and approach the epithelial surface anticipating attachment;
(3) cathelicidin mRNA levels rapidly rise, translation is activated, and peptide secreted rapidly, killing nearby bacteria. In this scenario, we permit some microbes to survive and continue to ascend; thus, (4) neutrophils begin to migrate toward the lumen; (5) neutrophils secrete cathelicidins, complementing epithelial sources, but viable microbes persist;
(6) neutrophils continue to invade the epithelium, resulting in cellular damage.
Michael Zasloff. Nature Medicine 12, 607 - 608 (2006)
|
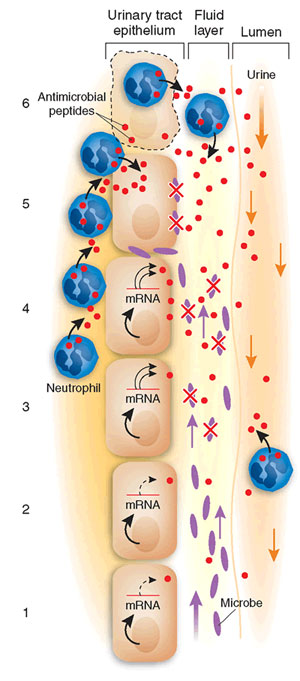
STRUCTURES OF HUMAN HOST DEFENSE CATHELICIDIN LL-37 AND ITS SMALLEST ANTIMICROBIAL PEPTIDE KR-12 IN LIPID MICELLES.
Guangshun Wang. JBC Papers in Press. Published on September 25, 2008 as Manuscript M805533200
The human cathelicidin anti-microbial protein, hCAP18 is a component of the innate immune system and has broad anti-microbial activity conferred by its C-terminal fragment LL-37. hCAP18 is constitutively produced in leukocytes and is induced in barrier organs upon inflammation and infection. We demonstrate here a novel role for this peptide in re-epithelialization of skin wounds. We show that high levels of hCAP18 are produced in skin in vivo upon wounding. The highest hCAP18 levels are attained at 48 h post-injury, declining to pre-injury levels upon wound closure. hCAP18 is detected in the inflammatory infiltrate and in the epithelium migrating over the wound bed. In chronic ulcers, however, hCAP18 levels are low and immunoreactivity for hCAP18/LL-37 is absent in ulcer edge epithelium. Using a noninflammatory ex vivo wound healing model, composed of organ-cultured human skin, we show that hCAP18 is strongly expressed in healing skin epithelium, and that treatment with antibodies raised and affinity purified against LL-37, inhibits re-epithelialization in a concentration-dependent manner. Immunoreactivity for the proliferation marker Ki67 is absent in the epithelium of such inhibited wounds, suggesting that LL-37 may play a part in epithelial cell proliferation. Thus, we suggest that, in addition to being an anti-microbial peptide, LL-37 also plays a part in wound closure and that its reduction in chronic wounds impairs re-epithelialization and may contribute to their failure to heal.
Heilborn JD, et al. J Invest Dermatol. 2003 Mar;120(3):379-89.
BACKGROUND: The innate immune system of human skin contains antimicrobial peptides known as cathelicidins (LL-37) and beta-defensins. In normal skin these peptides are negligible, but they accumulate in skin affected by inflammatory diseases such as psoriasis. We compared the levels of expression of LL-37 and human beta-defensin 2 (HBD-2) in inflamed skin from patients with atopic dermatitis and from those with psoriasis.
METHODS: The expression of LL-37 and HBD-2 protein in skin-biopsy specimens from patients with psoriasis, patients with atopic dermatitis, and normal subjects was determined by immunohistochemical analysis. The amount of antimicrobial peptides in extracts of skin samples was also analyzed by immunodot blot analysis (for LL-37) and Western blot analysis (for HBD-2). Quantitative, real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assays were used to confirm the relative expression of HBD-2 and LL-37 messenger RNA (mRNA) in the skin-biopsy specimens. These peptides were also tested for antimicrobial activity against Staphylococcus aureus with the use of a colony-forming assay.
RESULTS: Immunohistochemical analysis confirmed the presence of abundant LL-37 and HBD-2 in the superficial epidermis of all patients with psoriasis. In comparison, immunostaining for these peptides was significantly decreased in acute and chronic lesions from patients with atopic dermatitis (P=0.006 and P=0.03, respectively). These results were confirmed by immunodot blot and Western blot analyses. Real-time RT-PCR showed significantly lower expression of HBD-2 mRNA and LL-37 mRNA in atopic lesions than in psoriatic lesions (P=0.009 and P=0.02, respectively). The combination of LL-37 and HBD-2 showed synergistic antimicrobial activity by effectively killing S. aureus.
CONCLUSIONS: A deficiency in the expression of antimicrobial peptides may account for the susceptibility of patients with atopic dermatitis to skin infection with S. aureus.
Ong PY, et al. N Engl J Med 2002 Oct 10;347(15):1151-60
The mast cell is one of the major effector cells in inflammatory reactions and can be found in most tissues throughout the body. During inflammation, an increase in the number of mast cells in the local milieu occurs, and such accumulation requires directed migration of this cell population. As it has previously been reported that the human cathelicidin-derived antibacterial peptide, LL-37, stimulates the degranulation of mast cells, we hypothesized that LL-37 could be a mast cell chemotaxin. The present study shows that LL-37 is a potent chemotactic factor for mast cells. The chemotactic response was dose-dependent and bell-shaped, reaching an optimal concentration of 5 &mgr;g/ml. In addition, checkerboard analysis showed that cell migration towards this peptide was chemotactic rather than chemokinetic. Moreover, Scatchard analysis using 125I-labelled LL-37-derived peptide revealed that LL-37 has at least two classes of receptors, namely high- and low-affinity receptors, on mast cells. Furthermore, the competitive binding assay suggested that LL-37 is unlikely to utilize formyl peptide receptor-like 1 (FPRL1), a functional LL-37 receptor for neutrophil and monocyte migration, on mast cells. In addition, the treatment of cells with pertussis toxin and phospholipase C inhibitor, U-73122, inhibited LL-37-mediated migration, indicating that LL-37 induces mast cell chemotaxis through a Gi protein-phospholipase C signalling pathway. These results show that besides its antibacterial activities, LL-37 may have the potential to recruit mast cells to inflammation foci.
Niyonsaba F, et al. Immunology 2002 May;106(1):20-26
The antimicrobial activity of the collective molecules comprising human milk reflects an evolutionarily successful paradigm for preventing and limiting microbial infection. Understanding the molecules that participate in this process and how they work can yield insight into potentially new antimicrobial therapies. Upon proteolytic processing, antimicrobial peptides can be derived from milk proteins, such as lactoferrin, casein, and lysozyme. Similarly, using the HIV-1 gp41 protein template, we have demonstrated that the 28-residue C-terminus, when produced as an independent peptide, exhibits selective toxicity for bacteria over eukaryotic cells. Upon optimizing this sequence for cationic charge and hydrophobic character presented as a alpha-helical structure, we show improved capability of the parent LLP1 sequence to selectively kill bacteria in the host environment and that this activity is increased by the inclusion of Trp residues on the hydrophobic face. We report that it is possible to (i) design de novo antimicrobial peptides that demonstrate optimal antimicrobial activity with minimal inflammatory activity and (ii) design antimicrobial peptides to function in a defined environment. In the end, we describe a de novo designed antimicrobial peptide, WLBU2, which is selectively toxic to microbial pathogens in complex environments and does not stimulate a significant immunomodulatory response. In spite of these properties, WLBU2 activity against Pseudomonas aeruginosa in human milk is inferior to the host peptide LL37 with regard to antimicrobial potency. These studies demonstrate that antimicrobial peptides can be engineered for greater potency in one medium but may not be optimal for working in a different medium such as human milk.
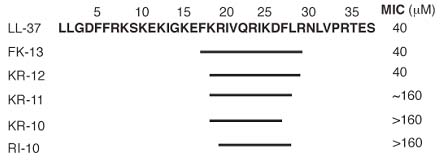
Human bronchial epithelial (HBE) cell stimulation of IL-8 production. HBE cells grown in
polarized culture were stimulated on the apical side with no peptide, LL37, or WLSA5.
Phadke SM, et al. J Nutr. 2005 May;135(5):1289-93.
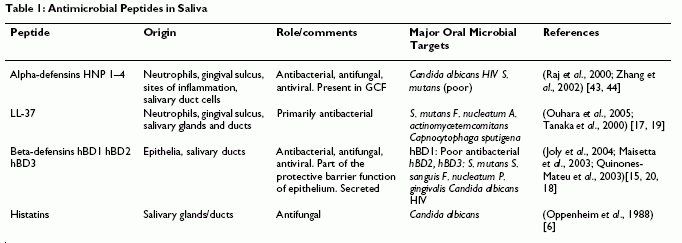
Dale BA, et al. BMC Oral Health. 2006 Jun 15;6 Suppl 1:S13.
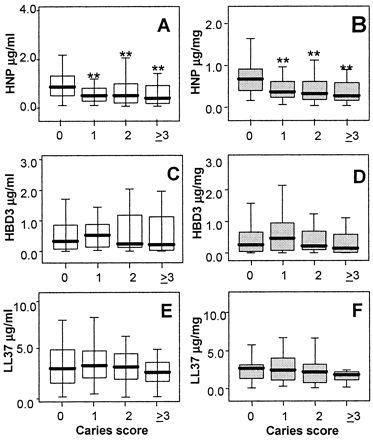
AMP levels in saliva as a function of caries score. (A, C, and E) HNP1-3 (A), hBD3 (B), and LL37 (C) concentrations in saliva, expressed as µg/ml; (B, D, and F) HNP1-3, hBD3, and LL37 levels relative to salivary protein in µg/mg protein. The caries-free group showed significantly higher HNP1-3 concentration (A) than each of the groups with caries.(**, P < 0.01) and significantly higher concentration than the combined caries groups (P = 0.004). Also, the HNP1-3 level relative to salivary protein (B) in the caries-free group is significantly higher than each of the caries groups (**, P < 0.01) and the combined caries groups (P < 0.004). For LL37, even though no significant difference was found among the groups with or without caries, the LL37 level relative to salivary protein (F) shows a trend of decreasing level in higher caries score groups. There is no evidence of association of hBD3 with caries (C and D). Box plots show the median and 25 to 75% range, with error bars indicating 5% and 95% intervals.
Tao R.C. et al, Antimicrobial Agents and Chemotherapy, September 2005, p. 3883-3888, Vol. 49, No. 9
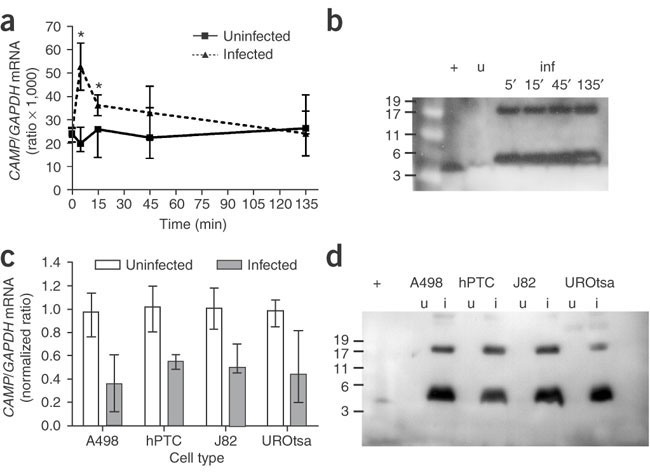
(a) mRNA encoding LL-37/hCAP-18 in uroepithelial
cells (UROtsa) without bacteria (continuous line) and after
infection with uropathogenic E. coli (dotted line).
All cell lines studied, both uroepithelial and renal epithelial,
reacted similarly after bacterial exposure. Data are presented
as median and range of CAMP/GAPDH X 1,000
mRNA ratios from two experiments in which each sample was
analyzed in triplicate. *P < 0.05. (b)
Western blot of supernatants from uroepithelial cells (UROtsa)
without bacteria (uninfected, u) and after 5, 15, 45 and
135 min infection with uropathogenic E. coli (infected,
inf). Similar results were obtained in all cell types studied,
both uroepithelial and renal epithelial. Synthetic LL-37
served as a positive control (+). Molecular weights are
in kDa. Discrepancy in the migration of the detected peptide
and the synthetic LL-37 is due to salt content in cell culture
medium (data not shown). (c) mRNA encoding
LL-37/hCAP-18 in renal epithelial cells (A498 and hPTC)
and uroepithelial cells (J82 and UROtsa) without bacteria
(open bars) and after 24-h infection with uropathogenic
E. coli (gray bars). mRNA levels from noninfected
cells are considered 1 and relative mRNA levels are calculated.
P < 0.01, compared with uninfected cells at
24 h. Data are presented as median and range of normalized
CAMP /GAPDH mRNA ratios from three independent
experiments. (d) Western blot of supernatants
from renal epithelial cells (A498 and hPTC) and uroepithelial
cells (J82 and UROtsa) without bacteria (uninfected, u)
and after 24-h infection with uropathogenic E. coli
(infected, i). Synthetic LL-37 served as a positive control
(+). Molecular weights are in kDa. Discrepancy in the migration
of the detected peptide and the synthetic LL-37 is due to
salt content in cell culture medium (data not shown).
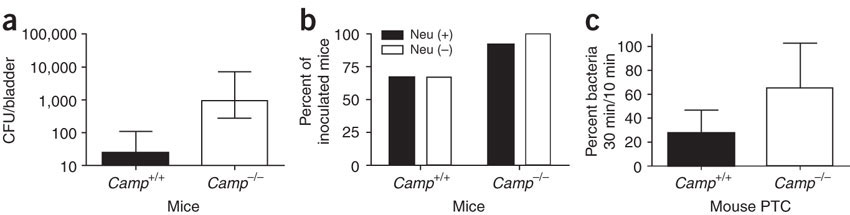
(a) Number of bacteria attached to urinary bladders of CRAMP-producing (Camp+/+) and CRAMP-deficient (Camp-/-) mice 1 h after bacterial inoculation. CFU, colony-forming units. Data are presented as median and range from one of two experiments with three to four mice per group. P < 0.05. (b) Infection rate of Camp+/+ and Camp-/- mice with (Neu (+)) or without neutrophils (Neu (–)). The infection rate was higher in Camp-/- mice, irrespective of the presence of neutrophils (P < 0.05). Data are presented as percentage of infected mice versus mice challenged with bacteria from three independent experiments using a total of 36 mice. (c) Survival of bacteria attached to Camp+/+ and Camp-/- mouse proximal tubular cells (Mouse PTC). Percentage of viable bacteria attached to the cells at 30 min of incubation in relation to the counts at 10 min is depicted. Data are presented as mean + s.d. from two independent experiments with nine samples per group. P < 0.05.
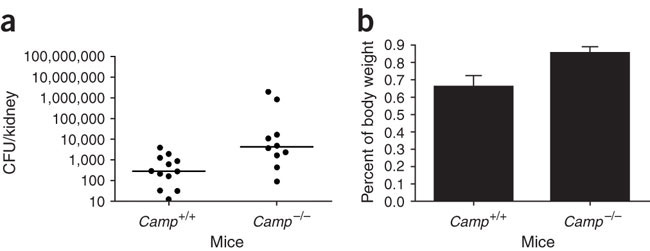
The course of experimental urinary tract infection in CRAMP-producing Camp+/+ and CRAMP-deficient Camp-/- mice. The number of bacteria in kidneys (a) and the size of kidneys (b) at 48 h of infection were higher in Camp-/- mice. P < 0.05. Accordingly, systemic response to infection was stronger in Camp-/- mice (data not shown). The differences were observed only in the presence of neutrophils and not after neutrophil depletion (data not shown). Three independent experiments were performed with five to six mice per group. Data are presented as medians with dot plots (a) and mean + s.d. (b) from representative experiments.
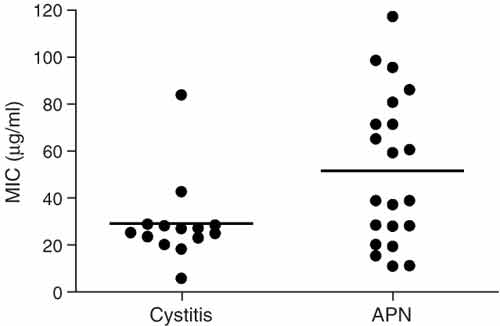
Sensitivity of uropathogenic E. coli to synthetic LL-37 peptide expressed as a minimal inhibitory concentration (MIC) of LL-37.Strains isolated from more invasive infection, pyelonephritis (APN, n = 21), were more resistant to LL-37 (P < 0.05) than strains isolated from infection of the lower urinary tract, cystitis (n = 14). Individual levels and means are shown.
| |
Antipseudomonal
activity of LL37 |
| |
EC50
(µM) against P. aeruginosa PAO1 |
| |
25
mM NaCI |
175
mM NaCI |
| LL37 |
0.81
± 0.13 |
1.47
± 0.33 |
|
Mean ± SE (n=3) |
|
| Relative
time for killing of P.aerginosa
PAO1 by LL37 |
| |
Concentration
(µM/ml) |
Half
time of killing (min) |
| LL37 |
36 |
62.0
± 26.3 |
|
Mean
± SE (n=3) |
|
| |
| |
Activity
of LL37 against gram-negative and
gram-positive bacteria |
| |
MIC
(µM) for: |
| |
E.coli
DH15a |
E.coli
ML-35p |
P.aeruginosa
PAO1 |
P.aeruginosa
MR3007 |
MRSA
ATCC33591 |
S.aureus
930918-3 |
| |
Low |
High |
Low |
High |
Low |
High |
Low |
High |
Low |
High |
Low |
High |
| LL37 |
0.19 |
0.44 |
1.20 |
0.58 |
0.54 |
1.03 |
0.53 |
1.54 |
5.45 |
30.8 |
3.58 |
2.58 |
|
| Low-salt
(no NaCI) and High-salt (100mM NaCI) |
|
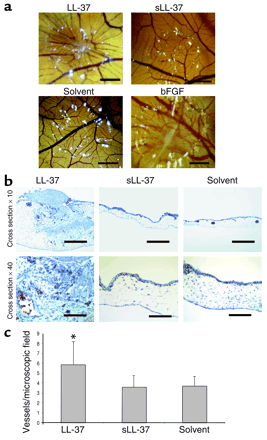
LL-37 induces physiologic angiogenesis in the CAM assay. (a) LL-37 (5 µg/pellet) induces the formation of wheel spoke–like vessel formation in comparison with the scrambled control peptide sLL-37 or the solvent. bFGF was used as positive control. Bars: 1 mm. (b) Cross sections of CAMs. LL-37 increases the number of erythrocyte-filled vessels (Masson-Goldner stain of erythrocyte-filled vessels). Bars: 250 µm or 62 µm in the x10 and x40 micrographs, respectively. (c) The numbers of erythrocyte-filled vessels were counted in hot spots. *P < 0.05 as compared with the solvent group (n = 6/group; three sections per CAM).
Koczulla R, J Clin Invest. 2003 Jun; 111 (11): 1665-72. |
| 
|
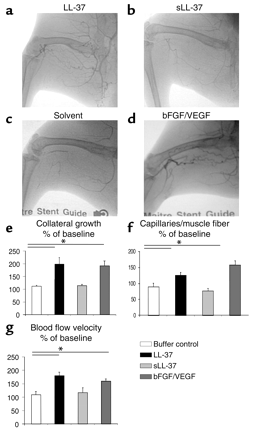
LL-37 induces angiogenesis and arteriogenesis in the rabbit hind-limb model. (a?b>d) Application of the peptide in a rabbit hind-limb model resulted in increased collateral growth in the LL-37–treated animals (a) as compared with the sLL-37–treated animals (b) or the buffer control group (c). bFGF/VEGF was used as positive control (d). (e) Collateral growth was significantly increased in the LL-37 group. (f) Tissue specimens from the calf (gastrocnemius muscle) revealed higher capillary density in the treated group as compared with the control group. (g) Blood flow velocity as assessed by cinedensitometry of the passage time of the contrast agent between the internal iliac artery and the anterior tibial artery was significantly augmented in the LL-37 group. *P < 0.05 as compared with the buffer control (n = 4/group).
Koczulla R, J Clin Invest. 2003 Jun; 111 (11): 1665-72.
|
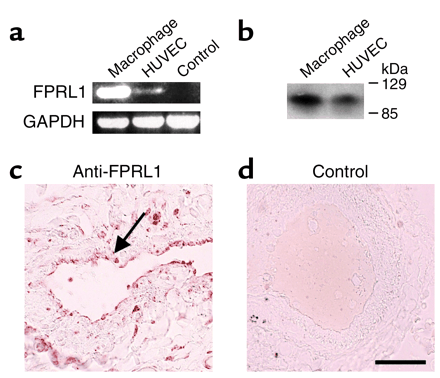
| Endothelial cells express FPRL1 in vitro and in vivo. (a) Detection of FPRL1 transcripts in cultivated HUVECs by RT-PCR. Control is the BEAS-2B cell line. (b) Detection of FPRL1 protein in HUVECs by Western blot analysis using a specific antiserum. (c) Immunohistochemistry revealed expression in endothelial cells (arrow) of sections of lung tissue. (d) Control serum revealed no positive staining. Bar: 60 µm.
Koczulla R, J Clin Invest. 2003 Jun; 111 (11): 1665-72. |
|
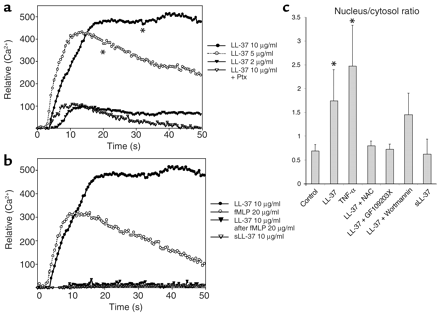
| LL-37 binds to endothelial FPRL1 and induces cellular signaling. (a and b) LL-37 induces Ca2+ flux in HUVECs. Fura-2–loaded HUVECs were stimulated with LL-37, and relative levels of intracellular Ca2+ were monitored using a calcium-imaging system. Local application of LL-37 led to a concentration-dependent increase of intracellular calcium. Preincubation of the cells with pertussis toxin (Ptx) resulted in partial inhibition of the Ca2+ flux (a). Preincubation with fMLP cross-desensitized the LL-37–induced Ca2+ mobilization; sLL-37 had no effect (b). (c) LL-37 activates NF-B in endothelial cells. This response can be inhibited by GF109203X and the addition of N-acetylcystein. *P < 0.05 as compared with the control group (n = 8). NAC, N-acetylcystein.
Koczulla R, J Clin Invest. 2003 Jun; 111 (11): 1665-72. |
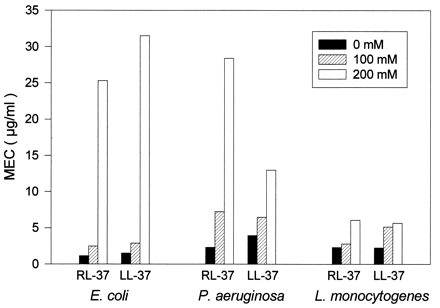
Activity against E. coli, P. aeruginosa, and L. monocytogenes. Radial diffusion assays were performed in underlay gels that contained different amounts of NaCl (0 mM, 100 mM, or 200 mM), in addition to their common basic ingredients (10 mM sodium phosphate buffer, pH 7.4; Trypticase soy broth powder, 0.3 mg/ml; and 1% [wt/vol] agarose).
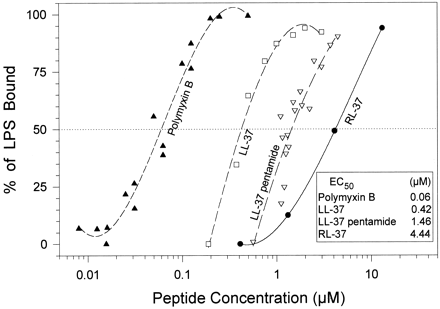
LPS binding. We used a chromogenic Limulus amoebocyte assay to obtain binding isotherms for E. coli 0111:B4 lipopolysaccharide. The peptides examined included polymyxin B, LL-37, LL-37 pentamide, RL-37, and rabbit CAP-18 (another LPS binding cathelicidin). The EC50 (i.e., the peptide concentrations that bound 50% of the LPS) are shown and provide an approximate binding constant.
| TABLE
3. Uffect of amidation on antimicrobial
activitya |
|
| Organism |
Strain |
P |
Mean
MEC (mM) ± SEM
|
| LL-37 |
LL-37pam |
RL-37 |
|
| Group
B strep |
A12973 |
0.019 |
8.37 ± 2.09 (8) |
2.27 ± 0.41 (7) |
>79.0 (3) |
| L.
acidophilus |
A4356 |
NS |
18.6 ± 6.1 (4) |
9.0 ± 1.7 (4) |
NT |
| K.
pneumoniae |
2270 |
0.02 |
1.81 ± 0.02 (9) |
0.76 ± 0.14 (7) |
0.73 ± 0.06 (3) |
| P.
aeruginosa |
MR3007 |
0.012 |
1.28 ± 0.18 (10) |
0.73 ± 0.05 (9) |
1.26 ± 0.08 (3) |
| E.
coli |
ML-35p |
NS |
0.66 ± 0.07 (9) |
0.61 ± 0.04 (6) |
0.57 ± 0.01 (3) |
| E.
coli |
A33780 |
NS |
5.57 ± 0.15 (3) |
6.47 ± 0.39 (3) |
5.74 ± 0.39 (3) |
|
a
Radial diffusion assays were performed with underlay
gels that contained 100 mM NaCl; 10 mM sodium
phosphate buffer, pH 7.4; and Trypticase soy broth
powder, 30 mg/ml. P values (for LL-37
versus LL-37 PAM) were calculated by the t
test. Abbreviations: NT, not tested; NS, not significant. |
|
|
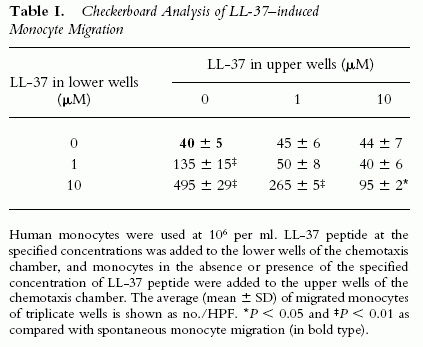
Induction by LL-37 of migration of (A) and Ca2+ flux in (B) human monocytes.
(A) The migration of monocytes (106 cells/ml) was assessed by chemotaxis assay using 5-µm uncoated membranes. Spontaneous cell migration (without LL-37) was 30–50 cells per HPF. The average C.I. (mean ± SD) of triplicate wells is shown. *P < 0.05 when compared with chemotaxis medium alone (open bar).
(B) Arrow indicates the time point at which LL-37 was applied to the cells.
Yang D., et al. The Journal of Experimental Medicine, Volume 192, Number 7, October 2, 2000 1069-1074 |
|
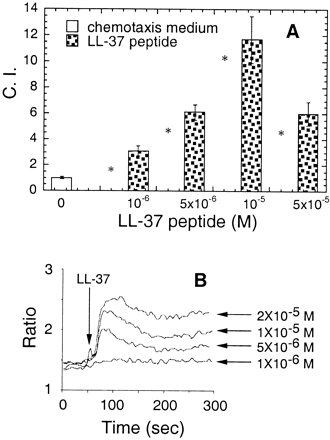
Effect of PTX (A) and serum (B) on LL-37–induced chemotaxis of monocytes. (A) Monocytes were incubated with (closed bar) or without (hatched bar) PTX at a final concentration of 200 ng/ml for 30 min at 37°C before performing chemotaxis assay. To show that the spontaneous cell migration (C.M.) was not affected by PTX pretreatment, the results are presented as no./HPF. (B) Chemotaxis assay was performed in the absence (hatched bar) or presence (closed bar) of 10% human AB serum, which can completely block the antimicrobial activity of LL-37 at 10-5 M.
Yang D., et al. The Journal of Experimental Medicine, Volume 192, Number 7, October 2, 2000 1069-1074 |
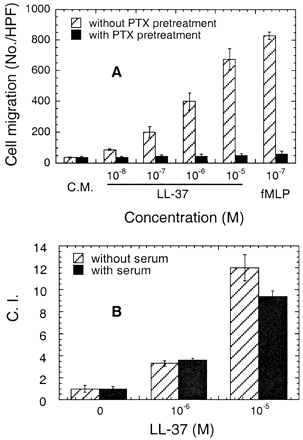
LL-37 uses FPRL1 as its receptor. (A) Selective induction of FPRL1/293 cell chemotaxis by LL-37. The migration of parental HEK293 (open bars), FPRL1/293 (dotted bars), or ETFR (closed bars) cells was assessed by chemotaxis assay with the use of collagen-coated 10-µm membranes. Cells were used at a concentration of 106 cells/ml. The average C.I. (mean ± SD) of triplicate wells is shown. Spontaneous cell migration (without LL-37) was 10–20 cells per HPF. *P < 0.05 when compared with chemotaxis medium alone. (B) LL-37–induced Ca2+ flux in FPRL1/293 cells. Arrow indicates the time point at which LL-37 was applied to the cells. (C) Cross-desensitization of LL-37–induced Ca2+ flux in monocytes by FPRL1-specific agonistic ligand. Arrows indicate the time points at which LL-37 and Su peptides were applied to the cells.
Yang D., et al. The Journal of Experimental Medicine, Volume 192, Number 7, October 2, 2000 1069-1074
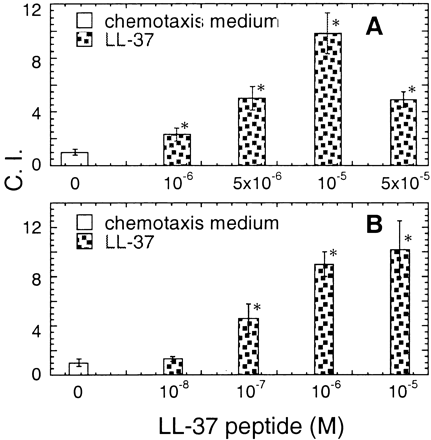
Chemotaxis of human neutrophils (A) and CD4 T lymphocytes (B) in response to LL-37. The cell migration was assessed by chemotaxis assay with the use of uncoated (A) or fibronectin-coated (B) 5-µm membranes. The results are presented as the average C.I. (mean ± SD) of triplicate wells. *P < 0.05 when compared with spontaneous cell migration (chemotactic medium alone). Neutrophils and CD4 T cells were used at a concentration of 106 cells/ml and 5 x 106 cells/ml, respectively. Spontaneous neutrophil and T cell migration (without LL-37) was 30–50 and 30–40 cells per HPF, respectively.
Yang D., et al. The Journal of Experimental Medicine, Volume 192, Number 7, October 2, 2000 1069-1074
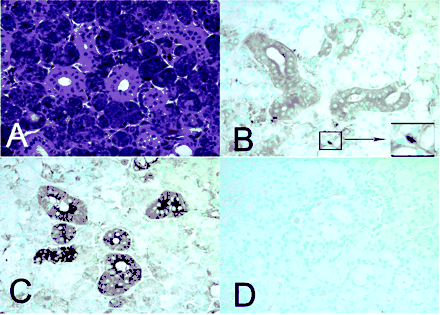
AMP expression in submandibular salivary gland. Immunohistochemistry for HNP1-3 and LL37 shows positive reactions in duct cells of submandibular glands. (A) Hematoxylin and eosin stain. (B) HNP1-3 immunostaining with monoclonal antibody D21 was positive in salivary duct cells and neutrophils (inset). (C) LL37 immunostaining was also positive in duct cells. (D) Negative control without primary antibody. An isotype-specific control for the HNP1-3 monoclonal antibody was also negative (not shown). Original magnification, x40.
Immunohistochemistry. Formalin-fixed sections were evaluated for expression of LL37 and HNP1-3 using the ABC technique. Briefly, sections were deparaffinized, rehydrated, and treated with antigen-unmasking solution (Vector Laboratories). Endogenous peroxide was blocked using 1% hydrogen peroxide/Tris-buffered saline for 30 min. Sections were blocked with appropriate sera and incubated with the primary antibody overnight before visualization with ABC reagents using 3,3'-diaminobenzidine as the substrate. Methyl green counterstain was used to visualize tissue morphology. The antibodies used were polyclonal rabbit anti-LL37 (Catalog No.: H-075-06) (Phoenix Pharmaceuticals, Inc., Belmont, CA) and monoclonal antibody clone D21 anti-HNP1-3. Histological sections of minor salivary glands were obtained from the Division of Oral Pathology, School of Dentistry, University of Washington, in accordance with Institutional Review Board procedures. Commercially available histological sections of human submandibular glands were from Spring Biosciences.
Unstimulated saliva was collected (3 to 5 ml),
the detergent Nonidet P-40 (Sigma, St. Louis, MO) was added
to a final concentration of 0.1%, and the sample was frozen
for later analysis. Saliva was thawed and cleared by centrifugation
twice at 15,000 rpm for 10 min. Total protein concentration
was evaluated in the supernatant (cleared unfractionated saliva)
by bicinchoninic acid assay (Pierce Inc., Rockford, IL). Cleared
unfractionated saliva was also used for the HNP1-3 enzyme-linked
immunosorbent assay according to the manufacturer's instructions
(HyCult Biotechnology, Uden, The Netherlands). Aliquots (200
µl) of supernatant were acid extracted by the addition
of an equal volume of 1 M HCl/1% trifluoroacetic acid overnight
with mixing in the cold (23). The sample
was centrifuged, and the supernatant was concentrated by vacuum
evaporation and resuspended in distilled water equal to the
starting sample volume. Acid-extracted saliva was used for
immunoassay of LL37 and hBD3. LL37 was assayed by slot blot
using an assay kit (Catalog No.: WBK-075-06) (Phoenix Pharmaceuticals
Inc., Belmont, CA). Statistical analysis for association of
AMP levels with caries score was done using the Kruskal-Wallis
nonparametric test based on rank and designed for nonnormally
distributed data.
Tao R.C. et al, Antimicrobial Agents and Chemotherapy, September 2005, p. 3883-3888, Vol. 49, No. 9
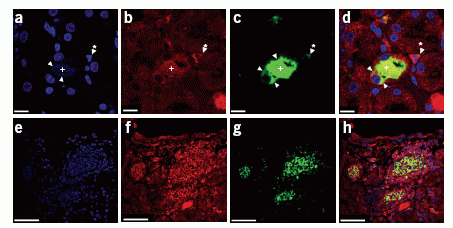
Sections are stained with propidium iodide (a,e,
blue), neutrophil-specific antibody (b,f,
red), antibody to CRAMP (c,g,
green), and images showing these three sets of panels merged
(d,h). The upper panel represents
marginal areas of infection corresponding to the early stage
of inflammation. Bacteria are stained in the lumen of renal
tubulus by propidium iodide (a, arrowheads)
and stimulate the production and release of CRAMP (c,
green, arrowheads). A neutrophil with typical segmented nucleus
(b, starred arrowhead) and positive for a
neutrophil marker (c, starred arrowhead)
is also positive for CRAMP (c,d,
starred arrowhead). Cross indicates the lumen of the renal
tubulus. The lower panel shows an area of massive infection
with abundance of bacteria, destruction of normal parenchyma
and accumulation of neutrophils (f) that
exhibit CRAMP immunoreactivity (g,h).
After blocking of antibody with synthetic CRAMP (1–39)
peptide, we did not observe any staining, and, likewise, there
was no staining in CRAMP-deficient mice (data not shown).
Scale bars in a–d,
20 µm; in e–h,
50 µm.
Chromek M., et al. Nature
Medicine 12, 636 - 641 (2006)
|
|
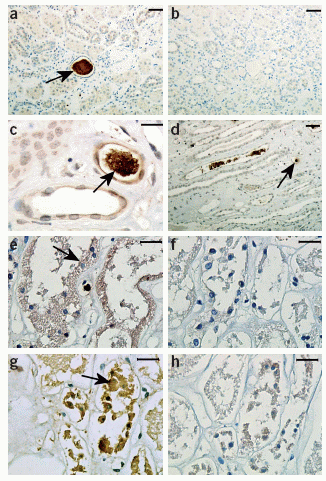
The sections are stained using polyclonal
antibody to LL-37 (a,c–e,g)
and normal rabbit immunoglobulin (b,f,h).
In the healthy renal tissue, we found LL-37/hCAP-18 immunoreactivity
in the hyaline substance in the lumen of renal tubuli (a,c,
brown) and in neutrophils (d) indicated by
arrows. Parallel sections of the kidney (a,b)
are shown. We did not observe any staining in the hyaline
substance when we used normal rabbit immunoglobulin instead
of primary antibody (b). After 24 h of in
vitro incubation, tubular epithelial cells displayed
morphological signs of cell death (e, arrow).
Moreover, in the presence of bacteria, epithelial cells stained
positive for LL-37/hCAP-18 (g, brown, arrow).
Parallel sections of the kidney (e–h)
are shown. We did not detect any staining when we used normal
rabbit immunoglobulin instead of primary antibody (f,h).
Scale bars in a, b and d,
50 µm; in c,
e, f, g
and h, 20 µm.
Chromek M., et al. Nature Medicine
12, 636 - 641
(2006)
|

Sequences of the WLBU. Hydrophobic residues are indicated by bold type.
Phadke SM, et al. J Nutr. 2005 May;135(5):1289-93
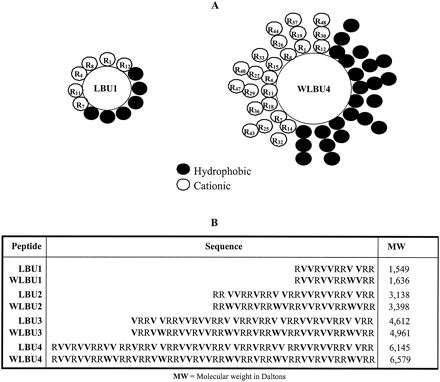
Peptide design. (A) The cationic amphipathic peptides were designed as demonstrated in helical wheel diagrams. Arg, Val, and Trp residues were arranged to form idealized amphipathic alpha-helices, with the hydrophilic and hydrophobic faces indicated in clear and shaded circles, respectively. The 12- and 48-mers LBU1 and WLBU4 are shown as representatives of the LBU and WLBU series, respectively. (B) Primary sequences of the LBU and WLBU peptides used in the present study. The shortest peptide forms one lytic base unit (LBU1) of 12 amino acids, and the others were designed as multimers (2, 3, and 4) of LBU1. The WLBU peptides were derived from the LBU series by substituting Trp residues at the indicated positions. Not shown here are the peptide hydrophobic moments calculated according to the method of Eisenberg et al. (J. Cell Biochem. 31:11-17.).
Deslouches B, et al. Antimicrob Agents Chemother. 2005 Jan;49(1):316-22.
|
Alignment
of RL-37, LL-37 and LL-37 pentamide
sequence |
|
| Peptide |
Primary
sequencea |
Avg
mass (Da) |
Net
charge |
|
| RL-37 |
RLGNFFRKVKEKIGGGLKKVGQKIKDFLGNLVPRTAS |
4,100.9 |
+8 |
| LL-37 |
LLGDFFRKSKEKIGKEFKRIVQRIKDFFRNLVPRTES |
4,527.3 |
+6 |
| LL-37
pentamide |
LLGNFFRKSKQKIGKQFKRIVQRIKNFFRNLVPRTQS |
4,522.3 |
+11 |
|
| a
The five amides that were introduced
into the LL-37 pentamide sequence
are shown in boldface type. Residues
of RL-37 that are identical to
those in LL-37 are underlined. |

cDNA sequence of the rhesus monkey cathelicidin, RL-37. The precursor has 170 residues, a mass of 18,861 Da, and a pI of 10.06. The predicted signal sequence (33 residues) is underlined, and the expected mature peptide is shown in bold face type. The stop codon is indicated by asterisks, and the polyadenylation site is indicated by italics.
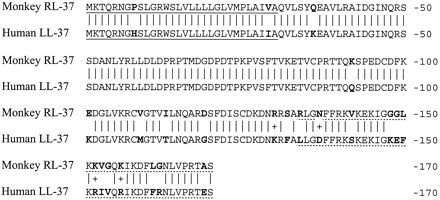
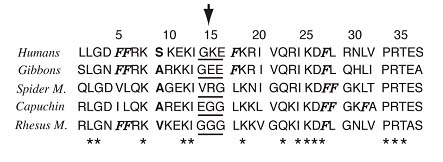
Comparison of rhesus and human cathelicidin peptides. Both signal peptides (underlined) have 30 residues, of which 28 (93.3%) are identical. Both cathelin domains have 101 residues, of which 93 (92%) are identical. The mature domains (dotted underlined) of RL-37 and LL-37 both contain 37 residues, of which 25 (67.6%) are identical. Mature rhesus RL-37 has a mass of 4,100.9 Da, a theoretical pI of 11.20, and a net charge of +8. Mature human LL-37 has a mass of 4527.34, a pI of 10.61, and a net charge of +6. A vertical line connects identical residues, and a plus sign identifies conservative substitutions. Residues that differ in the human and rhesus peptides are shown in boldface type.
Travis S.M., et al. Infection and Immunity. 68, 2748~2755 (2000)
Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002 May;106(1):20-26.
Le Y, Yang Y, Cui Y, Yazawa H, Gong W, Qiu C, Wang JM. Receptors for chemotactic formyl peptides as pharmacological targets. Int Immunopharmacol. 2002 Jan;2(1):1-13.
De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000 Oct 2;192(7):1069-74.
|
|
|
%LL37%;%075-28%
|
|
|


