Proglucagon is cleaved by PC1 at selected pairs of basic amino acids as well as at a single basic amino acid to liberate several biologically active peptides, including GLP-1. Note that GLP-1 normally also undergoes amidation, probably through the actions of peptidylglycine-{alpha}-amidating monooxygenase. The cleavage site for Ex-4 contains the consensus sequences for cleavage by furin, PC1, and/or PC2.
Adatia et al. Endocrinology. 2002 Sep;143(9):3464-71.
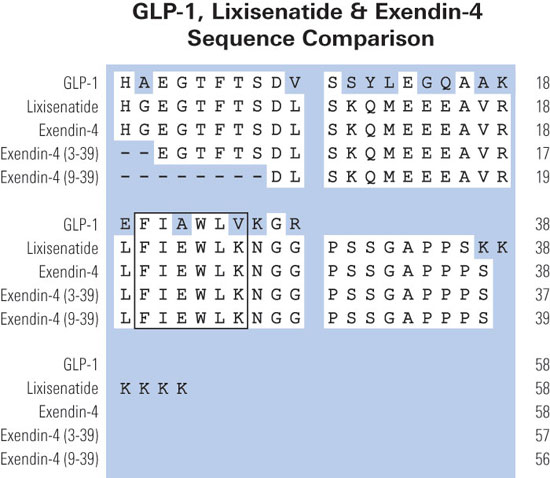
A, aligned amino acid sequences of several GLP-1R peptide ligands. B, schematic representation of the wild type rat GLP-1R (rGLP-1R) and the two truncated rat receptors used in this study, rNT-TM1 and 6H-rNT. The N-terminal domain is represented by gray ovals, and transmembrane helices are shown asgray cylinders. The expression of rGLP-1R and rNT-TM1 in a mammalian system is indicated by the putative glycosylation shown with the two Y-shaped symbols. The 6H-rNT protein was expressed in a bacterial system and lacks glycosylation but contains a His6 tag (HHHHHH) close to the N terminus.
López de Maturana et al. J Biol Chem. 2003 Mar 21;278(12):10195-200.
Glucagon-like peptide-1(7-36)amide (GLP-1) possesses several unique and beneficial effects for the potential treatment of type 2 diabetes. However, the rapid inactivation of GLP-1 by dipeptidyl peptidase IV (DPP IV) results in a short half-life in vivo (less than 2 min) hindering therapeutic development. In the present study, a novel His(7)-modified analogue of GLP-1, N-pyroglutamyl-GLP-1, as well as N-acetyl-GLP-1 were synthesised and tested for DPP IV stability and biological activity. Incubation of GLP-1 with either DPP IV or human plasma resulted in rapid degradation of native GLP-1 to GLP-1(9-36)amide, while N-acetyl-GLP-1 and N-pyroglutamyl-GLP-1 were completely resistant to degradation. N-acetyl-GLP-1 and N-pyroglutamyl-GLP-1 bound to the GLP-1 receptor but had reduced affinities (IC(50) values 32.9 and 6.7 nM, respectively) compared with native GLP-1 (IC(50) 0.37 nM). Similarly, both analogues stimulated cAMP production with EC(50) values of 16.3 and 27 nM respectively compared with GLP-1 (EC(50) 4.7 nM). However, N-acetyl-GLP-1 and N-pyroglutamyl-GLP-1 exhibited potent insulinotropic activity in vitro at 5.6 mM glucose (P<0.05 to P<0.001) similar to native GLP-1. Both analogues (25 nM/kg body weight) lowered plasma glucose and increased plasma insulin levels when administered in conjunction with glucose (18 nM/kg body weight) to adult obese diabetic (ob/ob) mice. N-pyroglutamyl-GLP-1 was substantially better at lowering plasma glucose compared with the native peptide, while N-acetyl-GLP-1 was significantly more potent at stimulating insulin secretion. These studies indicate that N-terminal modification of GLP-1 results in DPP IV-resistant and biologically potent forms of GLP-1. The particularly powerful antihyperglycaemic action of N-pyroglutamyl-GLP-1 shows potential for the treatment of type 2 diabetes.
Green et al. J Endocrinol. 2004 Mar;180(3):379-88.
OBJECTIVE: Glucagon-like peptide-1 (GLP-1) (7-36) amide is a glucoregulatory hormone with insulinotropic and insulinomimetic actions. We determined whether the insulinomimetic effects of GLP-1 are mediated through its principal metabolite, GLP-1 (9-36) amide (GLP-1m).
METHODS AND PROCEDURES: Glucose turnover during two, 2-h, euglycemic clamps was measured in 12 lean and 12 obese (BMI <25 or >30 kg/m(2)) male and female subject volunteers with normal oral glucose tolerance test. Saline or GLP-1m were infused from 0 to 60 min in each study. Additionally, seven lean and six obese subjects underwent a third clamp in which the GLP-1 receptor (GLP-1R) antagonist, exendin (9-39) amide was infused from -60 to 60 min with GLP-1m from 0 to 60 min.
RESULTS: No glucose infusion was required in lean subjects to sustain euglycemia (glucose clamp) during saline or GLP-1m infusions. However, in obese subjects glucose infusion was necessary during GLP-1m infusion alone in order to compensate for a marked (>50%) inhibition of hepatic glucose production (HGP). Plasma insulin levels remained constant in lean subjects but rose significantly in obese subjects after termination of the peptide infusions. During GLP-1R blockade, infusion of glucose was immediately required upon starting GLP-1m infusions in all subjects due to a more dramatic reduction in HGP, as well as a delayed and modest insulinotropic response.
DISCUSSION: We conclude that GLP-1m potently inhibits HGP and is a weak insulinotropic agent. These properties are particularly apparent and pronounced in obese but only become apparent in lean subjects during GLP-1 (7-36) receptor blockade. These previously unrecognized antidiabetogenic actions of GLP-1m may have therapeutic usefulness.
Elahi et al. Obesity (Silver Spring). 2008 Jul;16(7):1501-9.
Glucagon-like peptide-1 (GLP-1) is a potent stimulator of glucose-dependent insulin secretion. Exendin-4(1-39) (Ex-4), isolated from Gila monster venom, is a highly specific GLP-1 receptor agonist that exhibits a prolonged duration of action in vivo. Although the processing mechanisms underlying liberation of GLP-1 from its prohormone have been elucidated, those for Ex-4 remain unknown. To examine the requirements for proEx-4 processing in mammalian cells, BHK fibroblasts, InR1-G9 islet A cells, and AtT-20 corticotropes, which express different prohormone convertases (furin, prohormone convertase 2, and prohormone convertase 1, respectively) were transfected with full-length lizard proEx-4, and the processing of proexendin was examined by HPLC and RIA (n = 3). All of the transfected cell lines exhibited Ex-4-like immunoreactivity in the media, and Ex-4-like immunoreactivity was detected in extracts of InR1-G9 and AtT-20 cells. However, only media and extracts from AtT-20 cells (not InR1-G9 and BHK cells) contained a single peak by HPLC corresponding to synthetic Ex-4. To establish whether proEx-4 can be processed to Ex-4 in nonimmortalized mammalian cells in vivo, the molecular forms of exendin-4 were examined in mice expressing a metallothionein-proEx-4 transgene (n = 3-6 for both males and females). ProEx4 mRNA transcripts were detected by RT-PCR in a broad range of both endocrine and nonendocrine tissues. Ex-4-like immunoreactivity was detected in pituitary, fat, adrenals, and testes; however HPLC analyses demonstrated that processed Ex-4 was found only in adrenals and testes. These results indicate that lizard proEx-4 is processed to mature bioactive Ex-4 in both rodent endocrine and nonendocrine mammalian cell types in vitro and in murine tissues in vivo. These findings may be useful for engineering cells that express a lizard pro-Ex4 transgene for the treatment of type 2 diabetes.
Adatia et al. Endocrinology. 2002 Sep;143(9):3464-71.
Two fragments of the receptor for glucagon-like peptide-1 (GLP-1), each containing the N-terminal domain, were expressed and characterized in either bacterial or mammalian cells. The first fragment, rNT-TM1, included the N-terminal domain and first transmembrane helix and was stably expressed in the membrane of human embryonic kidney 293 cells. The second, 6H-rNT, consisted of only the N-terminal domain of the receptor fused with a polyhistidine tag at its N terminus. The latter fragment was expressed in Escherichia coli in the form of inclusion bodies from which the protein was subsequently purified and refolded in vitro. Although both receptor fragments displayed negligible (125)I-labeled GLP-1(7-36)amide-specific binding, they both displayed high affinity for the radiolabeled peptide antagonist (125) I-exendin-4(9-39). Competition binding studies demonstrated that the N-terminal domain of the GLP-1 receptor maintains high affinity for the agonist exendin-4 as well as the antagonists exendin-4(3-39) and exendin-4(9-39) whereas, in contrast, GLP-1 affinity was greatly reduced. This study shows that although the exendin antagonists are not dependent upon the extracellular loops and transmembrane helices for maintaining their normal high affinity binding, the endogenous agonist GLP-1 requires regions outside of the N-terminal domain. Hence, distinct structural features in exendin-4, between residues 9 and 39, provide additional affinity for the N-terminal domain of the receptor. These data are consistent with a model for the binding of peptide ligands to the GLP-1 receptor in which the central and C-terminal regions of the peptides bind to the N terminus of the receptor, whereas the N-terminal residues of peptide agonists interact with the extracellular loops and transmembrane helices.
López de Maturana et al. J Biol Chem. 2003 Mar 21;278(12):10195-200.
Glucagon-like peptide 1 (GLP-1) is a physiological stimulus of pancreatic beta-cell function. This enteroendocrine hormone is produced by intestinal L cells, and is delivered via the bloodstream to GLP-1 receptors (GLP-1Rs) on pancreatic beta-cells. In addition, there is evidence that beta-cell GLP-1Rs maintain sustained basal activity even in the absence of intestinal peptide, an observation that has raised the question whether these receptors have some degree of ligand-independent function. Here, we provide an alternative explanation for basal receptor activity based on our finding that biologically relevant amounts of fully processed GLP-1 are locally generated by insulinoma cell lines, as well as by alpha-cells of isolated rat islets in primary culture. Presence of GLP-1 was established by immunocytochemistry, as well as by selective ELISAs and bioassays of cell supernatants. A GLP-1R antagonist significantly reduced insulin secretion/production in beta-TC-6 insulinoma cells and isolated rat islets, suggesting a functionally important loop between locally produced GLP-1 and its cognate receptor. Treatment with this antagonist also inhibited the growth of beta-TC-6 cells. These observations provide novel insight into the function of insulin-producing cell lines and native beta-cells during in vitro culture, and they support the idea that locally produced GLP-1 may play a role in intra-islet regulation.
Masur et al. Mol Endocrinol. 2005 May;19(5):1373-82.

(A) three representations of GLP-1R ligands: exendin-4 (i), GLP-1 (ii), and exendin(9–39) (iii). For exendin-4 (i), the residue numbers at the junctions between the N-terminal region, central helical region, and C-terminal Trp-cage are shown. Although the other two peptides do not possess all three regions, the equivalent residue numbers are included in ii and iii. The conserved residues in the central region of all three peptides are on the same face of the helix, which is shaded to highlight this. (B) the interactions H, N, andEx between the peptides and receptor are shown for exendin-4 and GLP-1R (i), GLP-1 and GLP-1R (ii), and exendin(9–39) and the isolated N-terminal domain of GLP-1R (iii). The H interaction is common to all GLP-1R-ligand interactions and involves the binding of the N-terminal domain of the receptor to the conserved face of the putative central helix. The N interaction is common to all GLP-1R-agonist interactions and involves the interaction of the N-terminal region of the agonist with the extracellular loops and/or transmembrane regions of GLP-1R. The Ex interaction is common to all GLP-1R-exendin interactions and involves interaction between the N-terminal domain of the receptor and one or more of the unique structural features of exendin peptides (e.g. increased helicity, the Trp-cage, or the residues on the nonconserved face of the central helix). Exendin-4 can form all three interactions, whereas GLP-1 and exendin-4(9–39) can only form two because they lack either the C or N terminus present in exendin-4.
López de Maturana et al. J Biol Chem. 2003 Mar 21;278(12):10195-200.
Links to publications that use EK-070-94:
Kamei et al. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides.
J Control Release. 2009 Feb 27. [Epub ahead of print]
Samson et al. Gene therapy for diabetes: metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model.
Mol Ther. 2008 Nov;16(11):1805-12.
Links to publications that use RKU-070-94:
Huang et al. Preparation and characterization of a novel exendin-4 human serum albumin fusion protein expressed in Pichia pastoris.
J Pept Sci. 2008 May;14(5):588-95.


