|
|
| New peptides and kits derived from augurin/ECRG4 preprohormone |
The Esophageal Cancer Related Gene 4 (ECRG4) is a highly conserved tumour suppressor gene encoding various peptides (augurin, CΔ16 augurin, ecilin, argilin, CΔ16 argilin) which can be processed and secreted. In the present work, we examined ECRG4 expression and location in a wide range of rat organs and reviewed the available literature. ECRG4 mRNA was identified in all examined tissues by quantitative PCR (qPCR). ECRG4immunoreaction was mainly cytoplasmic, and was detected in heart and skeletal muscles, smooth muscle cells showing only weak reactions. In the digestive system, ECRG4 immunostaining was stronger in the esophageal epithelium, bases of gastric glands, hepatocytes and pancreatic acinar epithelium. In the lymphatic system, immunoreactive cells were detectable in the thymus cortex, lymph node medulla and splenic red pulp. In the central and peripheral nervous systems, different neuronal groups showed different reaction intensities. In the endocrine system, ECRG4immunoreaction was detected in the hypothalamic paraventricular and supraoptic nuclei, hypophysis, thyroid and parathyroid glands, adrenal zona glomerularis and medulla and Leydig cells, as well as in follicular and luteal cells of the ovary. In the literature, ECRG4 has been reported to inhibit cell proliferation and increase apoptosis in various cell types. It is down-regulated, frequently due to hypermethylation, in esophageal, prostate, breast and colon cancers, together with glioma (oncosuppressor function), although it is up-regulated in papillary thyroid cancer (oncogenic role).ECRG4 expression is also higher in non-proliferating cells of the lymphatic system. In conclusion, our identification of ECRG4 in many structures suggests the involvement of ECRG4 in the tumorigenesis of other organs and also the need for further research. In addition, on the basis of the location of ECRG4 in neurons and endocrine cells and the fact that it can be secreted, its role as a neurotransmitter/neuromodulator and endocrine factor must be examined in depth in the future.
** The authors of this article using Phoenix’s peptides Cat. # 012-19 and Cat.# 012-25 to identify other vendor’s polyclonal antibody lack of specificity to peptides in their studies
Porzionato A, Rucinski M, Macchi V et al., Eur J Histochem. 2015 May 18;59(2):2458. doi: 10.4081/ejh.2015.2458.
BACKGROUND: Human nasopharyngeal carcinoma (NPC) is a malignant type of cancer with an increasing incidence. As yet, however, molecular biomarkers with a strong diagnostic impact and a major therapeutic promise have remained elusive. Here, we identified the esophageal carcinoma related gene 4 (ECRG4) as a novel candidate tumor suppressor gene and a promising therapeutic target for NPC.
METHODS: RT-PCR, Western blotting, methylation-specific PCR and bisulfite sequencing were performed to assess the expression and methylation status of the ECRG4 gene in primary NPC samples, NPC-derived cell lines and patient-derived peripheral blood samples. The NPC-derived cell line CNE1 was selected for treatment with a methylation inhibitor to restore ECRG4 expression. In addition, cell proliferation, invasion and colony formation assays were performed to assess the inhibitory effects of exogenous ECRG4 expression in CNE1 cells.
RESULTS: Down-regulated ECRG4 expression was found to occur in 82.5% (33/40) of the primary NPC biopsies tested. This down-regulation was significantly correlated with its tumor-specific promoter methylation status (72.5%, 29/40) and was also observed in the matching peripheral blood samples from the NPC patients (57.5%, 23/40). Pharmacologic demethylation through 5-aza-dC treatment led to gene reactivation in ECRG4methylated and silenced NPC cell lines. Moreover, exogenous expression of ECRG4 in the CNE1 cell line strongly inhibited its growth and invasive capacities, as well as its enhanced chemosensitivity to cisplatin through autophagy induction.
CONCLUSION: Our data suggest that methylation-mediated suppression of the ECRG4 gene occurs frequently in NPC and that restoration of its expression may have therapeutic benefits.
You Y, Yang W, Qin X et al., Cell Oncol (Dordr). 2015 Jun;38(3):205-14. doi: 10.1007/s13402-015-0223-y. Epub 2015 Feb 24.
In humans, esophageal cancer-related gene 4 (ECRG4) is encoded by four exons in the c2orf40 locus of chromosome 2. Translation of ECRG4messenger ribonucleic acid produces a 148 amino acid-secreted 17 KDa protein that is then processed to 14, ten, eight, six, four, and two KDa peptides, depending on the cell in which the gene is expressed. As hypermethylation at the c2orf40 locus inhibits ECRG4 gene expression in many epithelial cancers, several investigators have speculated that ECRG4 is a candidate tumor suppressor. Indeed, overexpression of ECRG4 inhibits cell proliferation in vitro, but it also has a wide range of effects in vivo beyond its antitumor activity. ECRG4 overexpression affects apoptosis, senescence, cell migration, inflammation, injury, and infection responsiveness. ECRG4 activities also depend on its cellular localization, secretion, and post-translational processing. These cytokine/chemokine-like characteristics argue that ECRG4 is not a traditional candidate tumor suppressor gene, as originally predicted by its downregulation in cancer. We review how insights into the regulation of ECRG4 gene expression, knowledge of its primary structure, and the study of its emerging physiological functions come together to support a much more complex role for ECRG4 at the interface of inflammation, infection, and malignancy.
The authors has been used phoenix’s antibody for their cell straining in flow cytometry studies.
Baird A, Lee J, Podvin S et al., Gastrointest Cancer. 2014;2014(4):131-142.
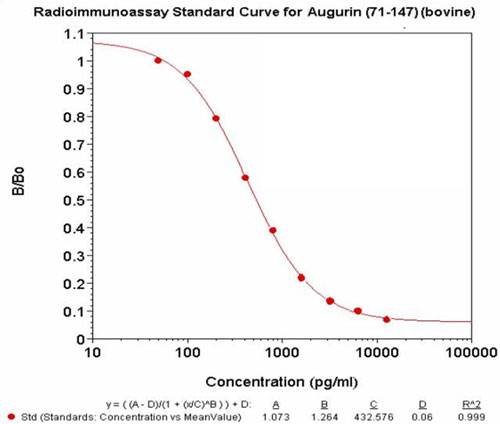
Peptide |
%Cross Reacitivity |
Augurin (71-147) (Bovine) |
100 |
Augurin (71-148) (Human)
|
100 |
Δ16/ Prepro-Augurin (133-148) (Human) |
100 |
ACTH (H,R,M), CRF (R,M) and AVP (H,R,M,O) |
0 |
|
|
|
augurin;new_augurin;32-EPS-Augurin-Poster
012-15;012-16;RK-012-27
|
|
|


