PURPOSE: To develop a mass spectrometry based scheduled reaction monitoring (SRM) assay for quantitation of Myeloid derived growth factor(MYGDF) formerly Chromosome 19 open reading frame (C19orf10) EXPERIMENTAL DESIGN: Candidate reporter peptides were identified in digests of recombinant MYDGF. Isotopically labelled forms of these reporter peptides were employed as internal standards for assay development. Two reference peptides were selected SYLYFTQFK and GAEIEYAMAYSK with respective limits of quantitation of 42 attomole and 380 attomole per injection.
RESULTS: Application of the assay to human serum and synovial fluid determined that the assay sensitivity was reduced and quantitation was not achievable. However, the partial depletion of albumin and immunoglobulin from synovial fluids provided estimates of 300-650 femtomoles per injection (0.7-1.6 nanomolar fluid concentrations) in three of the six sample analysed.
CONCLUSIONS AND CLINICAL RELEVANCE: A validated sensitive assay for the quantitation of MYDGF in biological fluids was developed. However, the endogenous levels of MYDGF in such fluids are at or below the current levels of quantitation. The levels of MYDGF are lower than those previously reported using an ELISA. The current results suggest that additional steps may be required to remove high abundance proteins or to enrich MYDGF for SRM based quantitation. This article is protected by copyright. All rights reserved.
Paracrine-acting proteins are emerging as a central mechanism by which bone marrow cell-based therapies improve tissue repair and heart function after myocardial infarction (MI). We carried out a bioinformatic secretome analysis in bone marrow cells from patients with acute MI to identify novel secreted proteins with therapeutic potential. Functional screens revealed a secreted protein encoded by an open reading frame on chromosome 19 (C19orf10) that promotes cardiac myocyte survival and angiogenesis. We show that bone marrow-derived monocytes and macrophages produce this protein endogenously to protect and repair the heart after MI, and we named it myeloid-derived growth factor (MYDGF). Whereas Mydgf-deficient mice develop larger infarct scars and more severe contractile dysfunction compared to wild-type mice, treatment with recombinant Mydgf reduces scar size and contractile dysfunction after MI. This study is the first to assign a biological function to MYDGF, and it may serve as a prototypical example for the development of protein-based therapies for ischemic tissue repair.
Korf-Klingebiel M, Reboll MR, Klede S et al., Nat Med. 2015 Jan 12. doi: 10.1038/nm.3778.
The identification of genes involved in tumor growth is crucial for the development of inventive anticancer treatments. Here, we have cloned a 17-kDa secretory protein encoded by c19orf10 from hepatocellular carcinoma (HCC) serial analysis of gene expression libraries. Gene expression analysis indicated that c19orf10 was overexpressed in approximately two-thirds of HCC tissues compared to the adjacent noncancerous liver tissues, and its expression was significantly positively correlated with that of alpha-fetoprotein (AFP). Overexpression of c19orf10 enhanced cell proliferation of AFP-negative HLE cells, whereas knockdown of c19orf10 inhibited cell proliferation of AFP-positive Hep3B and HuH7 cells along with G1 cell cycle arrest. Supplementation of recombinant c19orf10 protein in culture media enhanced cell proliferation in HLE cells, and this effect was abolished by the addition of antibodies developed against c19orf10. Intriguingly, c19orf10 could regulate cell proliferation through the activation of Akt/mitogen-activated protein kinase pathways. Taken together, these data suggest that c19orf10 might be one of the growth factors and potential molecular targets activated in HCC.
Sunagozaka H, Honda M, Yamashita T et al., Int J Cancer. 2011 Oct 1;129(7):1576-85. doi: 10.1002/ijc.25830. Epub 2011 Apr 13.
Joint inflammation and destruction have been linked to the deregulation of the highly synthetic fibroblast-like synoviocytes (FLSs), and much of our current understanding of the mechanisms that underlie synovitis has been collected from studies of FLSs. During a proteomic analysis of FLS cells, we identified a novel protein, c19orf10 (chromosome 19 open reading frame 10), that was produced in significant amounts by these cells. The present study provides a partial characterization of c19orf10 in FLSs, synovial fluid, and the synovium. Murine monoclonal and chicken polyclonal antibodies were produced against recombinant human c19orf10 protein and used to examine the distribution of c19orf10 in cultured FLSs and in synovial tissue sections from patients with rheumatoid arthritis or osteoarthritis. The intracellular staining pattern of c19orf10 is consistent with localization in the endoplasmic reticulum/Golgi distribution. Sections of rheumatoid arthritis and osteoarthritis synovia expressed similar patterns ofc19orf10 distribution with perivascular and synovial lining staining. Double-staining in situ analysis suggests that fibroblast-like synovial cells produced c19orf10, whereas macrophages, B cells, or T cells produced little or none of this protein. There is evidence of secretion into the vascular space and the extracellular matrix surrounding the synovial lining. A competitive enzyme-linked immunosorbent assay confirmed the presence of microgram levels of c19orf10 in the synovial fluids of patients with one of various arthropathies. Collectively, these results suggest that c19orf10 is an FLS-derived protein that is secreted into the synovial fluid. However, the significance of this protein in synovial biology remains to be determined. The absence of known structural motifs or domains and its relatively late evolutionary appearance raise interesting questions about its function.
Weiler T, Du Q, Krokhin O et al., Arthritis Res Ther. 2007;9(2):R30.
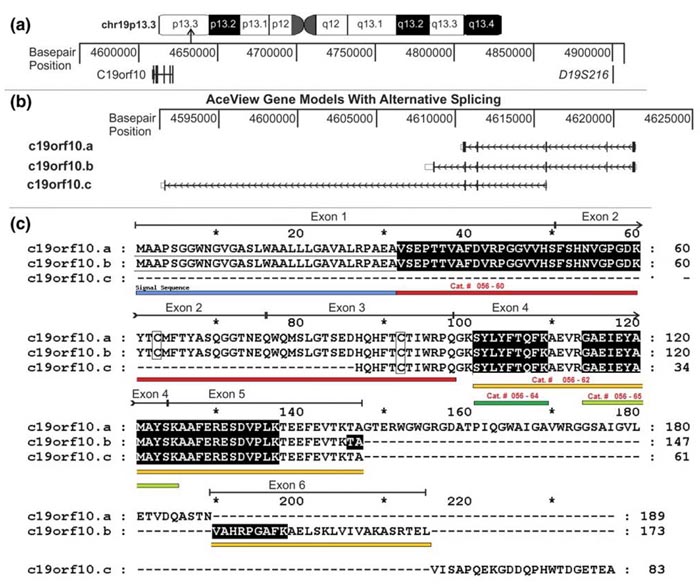
Genomic organization, alternative splicing, and protein sequence of c19orf10. (a) The chromosomal localization of the region containing the c19orf10 gene is indicated on the ideogram. The area containing the c19orf10 gene is expanded and the base-pair positions are indicated. The location of a microsatellite marker linked to juvenile rheumatoid arthritis, D19S216, is also indicated. (b) The expanded region of chromosome 19 containing the c19orf10 gene. Three putative splicing variants are indicated (c19orf10.a, c19orf10.b, and c19orf10.c). Thick blocks indicate translated exons, open blocks indicate untranslated exons, horizontal lines indicate introns, and the arrows indicate the direction of transcription. (c) Alignment of protein products of c19orf10 splicing variants. Variants a and b seem to be complete sequences starting with an N-terminal methionine. Variant c does not start with an N-terminal methionine and is probably incomplete at the N-terminus. Lines above the sequence map the exons to the protein sequence. Shaded sequences indicate peptides observed by mass spectrometry. The putative N-terminal signal peptide is underlined with a solid black line. C63 and 92 are indicated by rectangles. c19orf10, chromosome 19 open reading frame 10.
Figure & Text from: Weiler et al. Arthritis Res Ther. 2007;9(2):R30. doi:10.1186/ar2145