|
|
| DILP3 and DILP5-DB variant |
new peptides for the functional study in modeling metabolic disorders of the Drosophila cells |
See the perspectives in “Insulin Finds its Niche.” by Seth W. Cheetham, and Andrea H. Brand, Science 340, 817-818, May 17, 2013.
We report the crystal structure of two variants of Drosophila melanogaster insulin-like peptide 5 (DILP5) at a resolution of 1.85 Å. DILP5 shares the basic fold of the insulin peptide family (T conformation) but with a disordered B-chain C terminus. DILP5 dimerizes in the crystal and in solution. The dimer interface is not similar to that observed in vertebrates, i.e. through an anti-parallel β-sheet involving the B-chain C termini but, in contrast, is formed through an anti-parallel β-sheet involving the B-chain N termini. DILP5 binds to and activates the human insulin receptor and lowers blood glucose in rats. It also lowers trehalose levels in Drosophila. Reciprocally, human insulin binds to the Drosophila insulin receptor and induces negative cooperativity as in the human receptor. DILP5 also binds to insect insulin-binding proteins. These results show high evolutionary conservation of the insulin receptor binding properties despite divergent insulin dimerization mechanisms.

Functional studies of DILP5. Mitogenic potency is illustrated by [3H]thymidine incorporation of L6 rat muscle myoblasts stably transfected with IR-A (A). L6 rat muscle myoblast were treated with increasing concentrations of human insulin (.), C4 (×), and DB (+). The results are illustrated as percent of the insulin response at the highest concentration (10−6 m) for each assay. Curves are the average of at least three assays, each made in duplicate, and are fitted using a sigmoidal dose-response curve fit with variable slope. Panel B depicts glucose uptake in primary rat adipocytes as a function of increasing concentrations of ligand. [3-3H]Glucose uptake was measured by incubating freshly isolated rat adipocytes with increasing concentrations of human insulin (.), C4 (×), and DB (+). Curves are representative of three assays, each made in duplicate. Panel C shows intravenous administration of human insulin and DB in serum-starved Sprague-Dawley rats. The rats were anesthetized before intravenous injection of the ligands. Injection of human insulin 2.5 nmol/kg (.) and DB 500 nmol/kg (+) was performed at time point 0, and 10-μl blood samples were collected from the tail tip 10 time points in addition to a time point before injection. Panel D shows the effect of injection of DB on trehalose level in Drosophila, Five-day-old female flies were injected with 0.01 mg/ml DB. The mean and S.D. are plotted from samples collected before injection (0 min) and at 5, 10, 20, 30, and 40 min. Trehalose is per 2.5 μl of sample homogenate. p < 0.05 (*) and p < 0.01 (**) compared with 0 min (one-way analysis of variance with Dunnett's post-test).
Sajid W, Kulahin N, Schluckebier G et al., J Biol Chem. 2011 Jan 7;286(1):661-73. doi: 10.1074/jbc.M110.156018. Epub 2010 Oct 25.
A set of 14 insulin-producing cells (IPCs) in the Drosophila brain produces three insulin-like peptides (DILP2, 3 and 5). Activity in IPCs and release of DILPs is nutrient dependent and controlled by multiple factors such as fat body-derived proteins, neurotransmitters, and neuropeptides. Two monoamine receptors, the octopamine receptor OAMB and the serotonin receptor 5-HT1A, are expressed by the IPCs. These receptors may act antagonistically on adenylate cyclase. Here we investigate the action of the two receptors on activity in and output from the IPCs. Knockdown of OAMB by targeted RNAi led to elevated Dilp3 transcript levels in the brain, whereas 5-HT1A knockdown resulted in increases of Dilp2 and 5. OAMB-RNAi in IPCs leads to extended survival of starved flies and increased food intake, whereas 5-HT1A-RNAi produces the opposite phenotypes. However, knockdown of either OAMB or 5-HT1A in IPCs both lead to increased resistance to oxidative stress. In assays of carbohydrate levels we found that 5-HT1A knockdown in IPCs resulted in elevated hemolymph glucose, body glycogen and body trehalose levels, while no effects were seen after OAMB knockdown. We also found that manipulations of the two receptors in IPCs affected male aggressive behavior in different ways and 5-HT1A-RNAi reduced courtship latency. Our observations suggest that activation of 5-HT1A and OAMB signaling in IPCs generates differential effects on Dilp transcription, fly physiology, metabolism and social interactions. However the findings do not support an antagonistic action of the two monoamines and their receptors in this particular system.
Luo J, Lushchak OV1, Goergen P2, Williams MJ2, Nässel DR1. PLoS One. 2014 Jun 12;9(6):e99732. doi: 10.1371/journal.pone.0099732. eCollection 2014.
The neuroendocrine architecture and insulin/insulin-like signaling (IIS) events in Drosophila are remarkably conserved. As IIS pathway governs growth and development, metabolism, reproduction, stress response, and longevity; temporal, spatial, and nutrient regulation of dilps encoding Drosophila insulin-like peptides (DILPs) provides potential mechanisms in modulating IIS. Of eight DILPs (DILP1-8) identified, recent studies have furthered our understanding of physiological roles of DILP2, DILP3, DILP5, and DILP6 in metabolism, aging, and responses to dietary restriction (DR), which will be the focus of this review. While the DILP producing IPCs of the brain secrete DILP2, 3, and 5, fat body produces DILP6. Identification of factors that influence dilp expression and DILP secretion has provided insight into the intricate regulatory mechanisms underlying transcriptional regulation of those genes and the activity of each peptide. Studies involving loss-of-function dilp mutations have defined the roles of DILP2 and DILP6 in carbohydrate and lipid metabolism, respectively. While DILP3 has been implicated to modulate lipid metabolism, a metabolic role for DILP5 is yet to be determined. Loss of dilp2 or adult fat body specific expression of dilp6 has been shown to extend lifespan, establishing their roles in longevity regulation. The exact role of DILP3 in aging awaits further clarification. While DILP5 has been shown associated with DR-mediated lifespan extension, contradictory evidence that precludes a direct involvement of DILP5 in DR exists. This review highlights recent findings on the importance of conserved DILPs in metabolic homeostasis, DR, and aging, providing strong evidence for the use of DILPs in modeling metabolic disorders such as diabetes and hyperinsulinemia in the fly that could further our understanding of the underlying processes and identify therapeutic strategies to treat them.
Kannan K, Fridell YW. Front Physiol. 2013 Oct 16;4:288. doi: 10.3389/fphys.2013.00288.

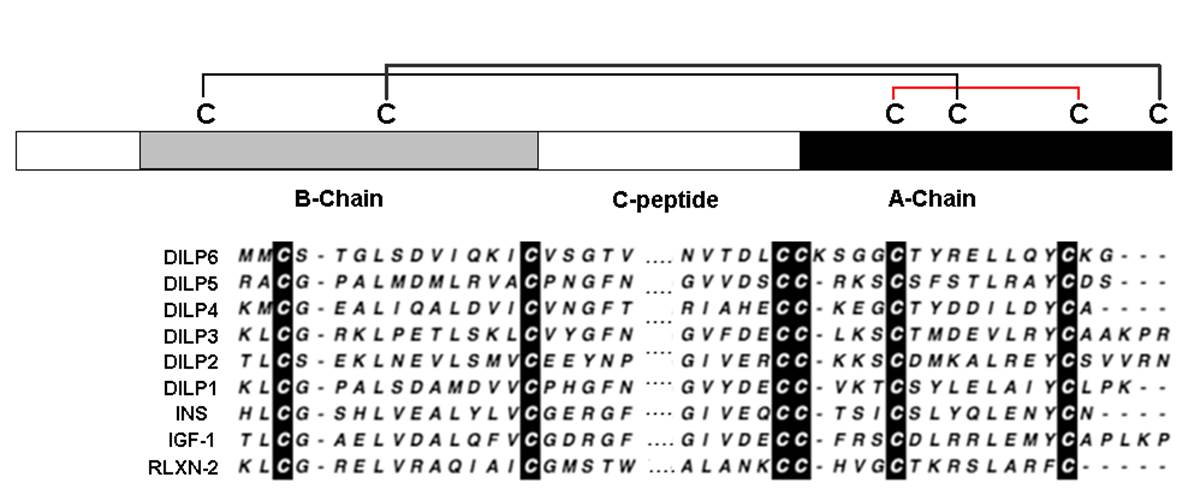
Schematic diagram of the disulfide bridges structure and the alignments of the predicted A and B chains of DILP6 with the five previously characterized Drosophila ILPs and representative members of the human insulin-like family of peptides.
|
|
|
%DILP%
%DILP%
|
|
|


