|
Computational methods have led two groups to predict the endogenous presence of a highly conserved, amidated, 14-aa neuropeptide called either spexin or NPQ. NPQ/spexin is part of a larger prohormone that contains 3 sets of RR residues, suggesting that it could yield more than one bioactive peptide; however, no in vivo activity has been demonstrated for any peptide processed from this precursor. Here we demonstrate biological activity for two peptides present within proNPQ/spexin. NPQ/spexin (NWTPQAMLYLKGAQ-NH(2)) and NPQ 53-70 (FISDQSRRKDLSDRPLPE) have differing renal and cardiovascular effects when administered intracerebroventricularly or intravenously into rats. Intracerebroventricular injection of NPQ/spexin produced a 13 ± 2 mmHg increase in mean arterial pressure, a 38 ± 8 bpm decrease in heart rate, and a profound decrease in urine flow rate. Intracerebroventricular administration of NPQ 53-70 produced a 26 ± 9 bpm decrease in heart rate with no change in mean arterial pressure, and a marked increase in urine flow rate. Intraventricular NPQ/spexin and NPQ 53-70 also produced antinociceptive activity in the warm water tail withdrawal assay in mice (ED(50)< 30 and 10 nmol for NPQ/spexin and NPQ 53-70, respectively). We conclude that newly identified peptides derived from the NPQ/spexin precursor contribute to CNS-mediated control of arterial blood pressure and salt and water balance and modulate nociceptive responses.-Toll, L., Khroyan, T. V., Sonmez, K., Ozawa, A., Lindberg, I., McLaughlin, J. P., Eans, S. O., Shahien, A. A., Kapusta, D. R. Peptides derived from the prohormone proNPQ/spexin are potent central modulators of cardiovascular and renal function and nociception.
Toll L., et al., FASEB J. 2011 Oct 28. [Epub ahead of print]
Spexin is a highly conserved peptide which was recently identified through the bioinformatics approach. Immunohistochemical analysis of its expression has not yet been performed. Thus, in this study, we examined spexin location in a wide range of rat organs by both RT-PCR and IHC. RT-PCR identified spexin mRNA in all tissues examined. Spexin immunoreaction was mainly cytoplasmic. Spexin was immunohistochemically detected, although with different staining intensities, in epithelia and glands of skin and respiratory, digestive, urinary, and reproductive systems. Smooth muscle cells showed weak immunostaining, and connective tissue was negative. In the central nervous system, neuronal groups showed different intensities for reaction product. Immunoreaction was also found in ganglionic cells of both trigeminal and superior cervical ganglia and in photoreceptor, inner nuclear, and ganglionic layers of the retina. In the endocrine system, spexin immunoreaction was detected in the hypothalamic paraventricular and supraoptic nuclei; adenohypophysis, thyroid, and parathyroid glands; adrenal cortex and medulla (mainly ganglionic cells); Leydig cells; and thecal, luteal, and interstitial cells of the ovary. Because of its widespread expression, spexin is probably involved in many different physiological functions; in particular, location of spexin in neurons and endocrine cells suggests its roles as neurotransmitter/neuromodulator and endocrine factor.
Porzionato A., et al., J. Histochem Cytochem. 2010 Sep;58(9):825-37. Epub 2010 Jun 7.
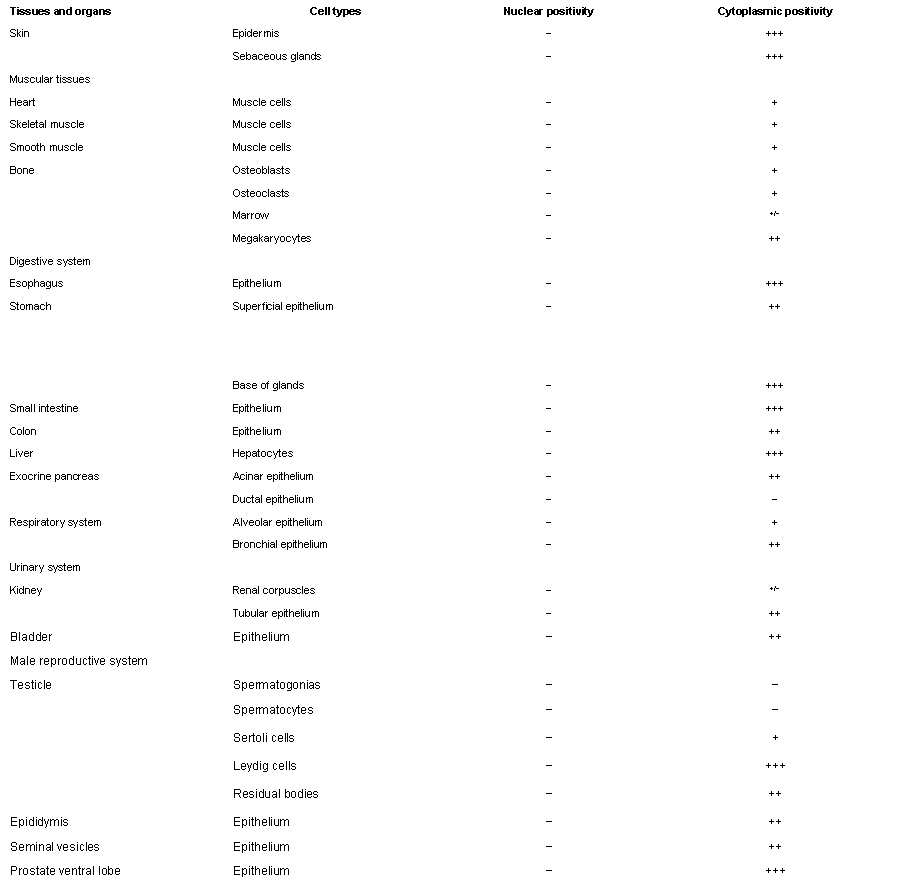
Porzionato A., et al., J. Histochem Cytochem. 2010 Sep;58(9):825-37. Epub 2010 Jun 7.
*This reference uses the Phoenix antibody for staining.
Spexin (SPX, also called NPQ) is a recently identified, highly conserved peptide which is processed and secreted. We analysed the SPX gene and its protein product in the rat adrenal gland to ascertain whether SPX is involved in the regulation of corticosteroid secretion of and growth of adrenocortical cells. In adult rat adrenal glands the highest levels of SPX mRNA were present in the glomerulosa (ZG) and fasciculate/reticularis (ZF/R) zones. High SPX gene expression levels were found in freshly isolated adult rat ZG and ZF/R cells. In cultured adrenocortical cells the levels of SPX mRNA were lower than in freshly isolated cells. SPX mRNA expression levels were found to be 2-3 times higher during days 90-540 of postnatal development than found during days 2-45. Prolonged ACTH administration lowered and dexamethasone increased adrenal SPX mRNA levels in vivo. Adrenal enucleation produced a significant linear increase in SPX mRNA levels, with the highest value occurring at day 8 after surgery, with control values taken on day 30 after enucleation. Immunohistochemistry revealed SPX-like immunoreactivity in the entire cortex of the adult male rat and in enucleation-induced regenerating cortex. A concentration of 10-6M SPX peptide stimulated basal aldosterone secretion by freshly isolated ZG. In prolonged exposure of adrenocortical cell primary cultures to SPX (10-6M) resulted in a small increase in corticosterone secretion and a notable decrease in BrdU incorporation. The results suggest the direct involvement of SPX in the regulation of adrenocortical cell proliferation; however, the mechanism of action remains unknown.
Rucinski, M. et al., Peptides. 2010 Apr;31(4):676-82. Epub 2010 Jan 4.
There are currently a large number of "orphan" G-protein-coupled receptors (GPCRs) whose endogenous ligands (peptide hormones) are unknown. Identification of these peptide hormones is a difficult and important problem. We describe a computational framework that models spatial structure along the genomic sequence simultaneously with the temporal evolutionary path structure across species and show how such models can be used to discover new functional molecules, in particular peptide hormones, via cross-genomic sequence comparisons. The computational framework incorporates a priori high-level knowledge of structural and evolutionary constraints into a hierarchical grammar of evolutionary probabilistic models. This computational method was used for identifying novel prohormones and the processed peptide sites by producing sequence alignments across many species at the functional-element level. Experimental results with an initial implementation of the algorithm were used to identify potential prohormones by comparing the human and non-human proteins in the Swiss-Prot database of known annotated proteins. In this proof of concept, we identified 45 out of 54 prohormones with only 44 false positives. The comparison of known and hypothetical human and mouse proteins resulted in the identification of a novel putative prohormone with at least four potential neuropeptides. Finally, in order to validate the computational methodology, we present the basic molecular biological characterization of the novel putative peptide hormone, including its identification and regional localization in the brain. This species comparison, HMM-based computational approach succeeded in identifying a previously undiscovered neuropeptide from whole genome protein sequences. This novel putative peptide hormone is found in discreet brain regions as well as other organs. The success of this approach will have a great impact on our understanding of GPCRs and associated pathways and help to identify new targets for drug development.
Sonmez K, et al. PLoS Comput Biol. 2009 Jan;5(1):e1000258. Epub 2009 Jan 9.
Peptide hormones are small, processed, and secreted peptides that signal via
membrane receptors and play critical roles in normal and pathological
physiology. The search for novel peptide hormones has been hampered by their
small size, low or restricted expression, and lack of sequence similarity. To
overcome these difficulties, we developed a bioinformatics search tool based on
the hidden Markov model formalism that uses several peptide hormone sequence
features to estimate the likelihood that a protein contains a processed and
secreted peptide of this class. Application of this tool to an alignment of
mammalian proteomes ranked 90% of known peptide hormones among the top 300
proteins. An analysis of the top scoring hypothetical and poorly annotated
human proteins identified two novel candidate peptide hormones. Biochemical
analysis of the two candidates, which we called spexin and augurin, showed that
both were localized to secretory granules in a transfected pancreatic cell line
and were recovered from the cell supernatant. Spexin was expressed in the
submucosal layer of the mouse esophagus and stomach, and a predicted peptide from
the spexin precursor induced muscle contraction in a rat stomach explant assay.
Augurin was specifically expressed in mouse endocrine tissues, including
pituitary and adrenal gland, choroid plexus, and the atrio-ventricular node of
the heart. Our findings demonstrate the utility of a bioinformatics approach to
identify novel biologically active peptides. Peptide hormones and their
receptors are important diagnostic and therapeutic targets, and our results
suggest that spexin and augurin are novel peptide hormones likely to be
involved in physiological homeostasis.
Mirabeau O, et al. Genome Res. 2007 Mar;17(3):320-7. Epub 2007 Feb 6.
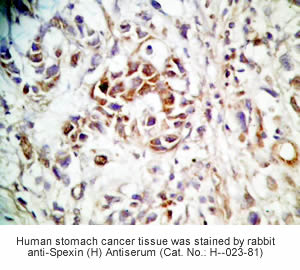 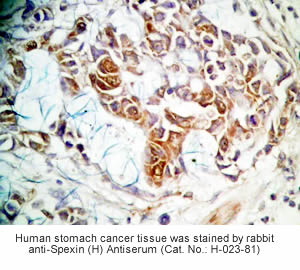
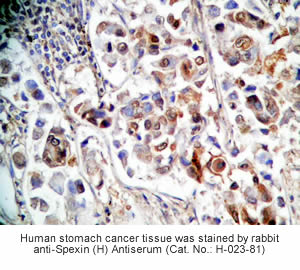 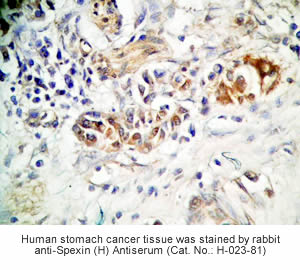
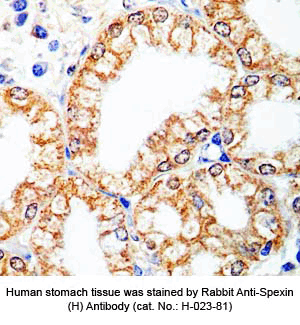 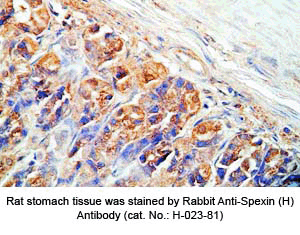
Tissue Sample | Human stomach tissues, stomach cancer tissues and rat stomach tissues |
Fixative | 10% formalin |
Embedding | Paraffin |
Negative Control | No primary antibody |
Pretreatment | N/A |
Blocking | 3% H2O2, 2% Normal Goat Serum |
Primary Antibody | Rabbit Anti-Spexin (H) Antiserum
(Catalog No.: H-023-81) |
Optimal Dilution | 1:500, 1 hour at RT |
Secondary Antibody | Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification | Streptavidin-HRP (Vector), 1:400, 30 min |
Detection System | HRP |
Substrate | DAB (Sigma), 3 min |
Counterstained | Hematoxylin, 30 sec |
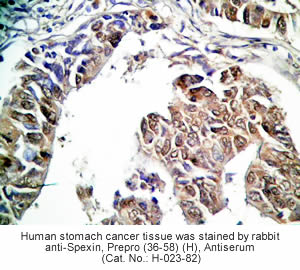 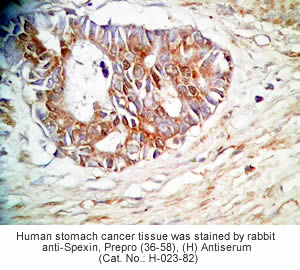
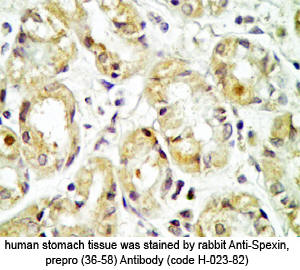 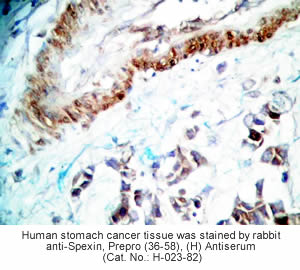
Tissue Sample | Human stomach tissues and stomach cancer tissues |
Fixative | 10% formalin |
Embedding | Paraffin |
Negative Control | No primary antibody |
Pretreatment | N/A |
Blocking | 3% H2O2, 2% Normal Goat Serum |
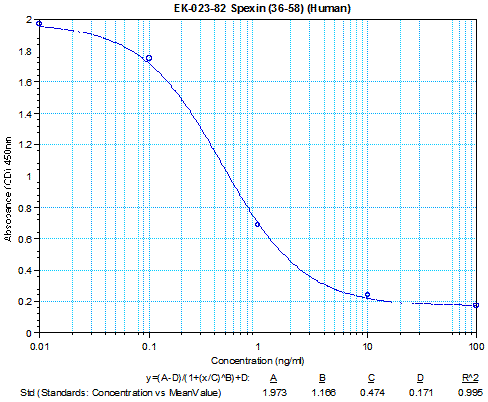
Primary Antibody | Rabbit Anti-Spexin, Prepro (36-58) (H) Antiserum
(Catalog No.: H-023-82) |
Optimal Dilution | 1:500, 1 hour at RT |
Secondary Antibody | Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification | Streptavidin-HRP (Vector), 1:400, 30 min |
Detection System | HRP |
Substrate | DAB (Sigma), 3 min |
Counterstained | Hematoxylin, 30 sec |
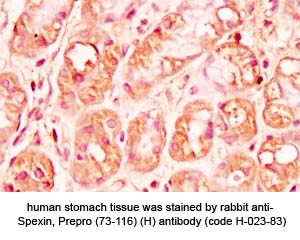
Tissue Sample | Human stomach tissues and stomach cancer tissues |
Fixative | 10% formalin |
Embedding | Paraffin |
Negative Control | No primary antibody |
Pretreatment | N/A |
Blocking | 3% H2O2, 2% Normal Goat Serum |
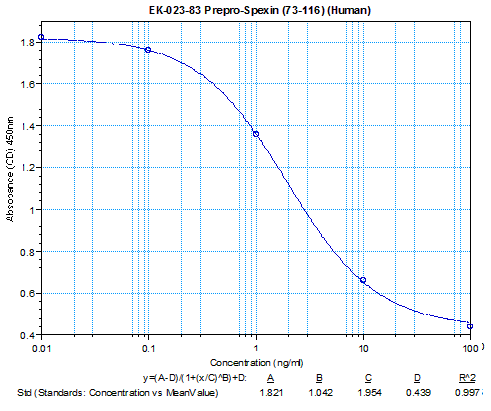
Primary Antibody | Rabbit Anti-Spexin, Prepro (73-116) (H) Antiserum
(Catalog No.: H-023-83) |
Optimal Dilution | 1:500, 1 hour at RT |
Secondary Antibody | Goat Anti-Rabbit IgG, Biotinylated (1:400), 30 min |
Amplification | Streptavidin-HRP (Vector), 1:400, 30 min |
Detection System | HRP |
Substrate | DAB (Sigma), 3 min |
Counterstained | Hematoxylin, 30 sec |
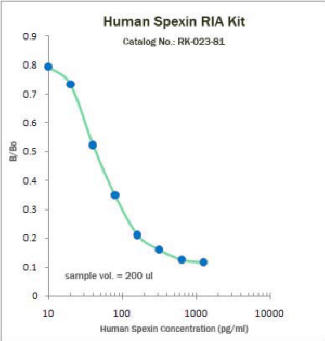
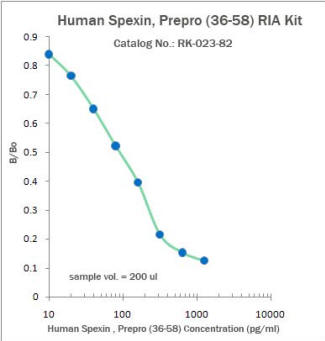
|


