Ciliary
NeuroTrophic Factor (CNTF)
Neurogenesis in the Hypothalamus of Adult Mice: Potential
Role in Energy Balance.
Ciliary neurotrophic factor (CNTF) induces weight loss in obese
rodents and humans, and for reasons that are not understood,
its effects persist after the cessation of treatment. Here we
demonstrate that centrally administered CNTF induces cell proliferation
in feeding centers of the murine hypothalamus. Many of the newborn
cells express neuronal markers and show functional phenotypes
relevant for energy-balance control, including a capacity for
leptin-induced phosphorylation of signal transducer and activator
of transcription 3 (STAT3). Coadministration of the mitotic
blocker cytosine-ß-D-arabinofuranoside (Ara-C) eliminates the
proliferation of neural cells and abrogates the long-term, but
not the short-term, effect of CNTF on body weight. These findings
link the sustained effect of CNTF on energy balance to hypothalamic
neurogenesis and suggest that regulated hypothalamic neurogenesis
in adult mice may play a previously unappreciated role in physiology
and disease.
Maia V. Kokoeva, Huali Yin, Jeffrey S. Flier. Science 28 October
2005: Vol. 310. no. 5748, pp. 679 - 683, DOI: 10.1126/science.1115360
The Regulation and Activation of Ciliary Neurotrophic
Factor Signaling Proteins in Adipocytes
Zvonic S. et al. J. Biol. Chem., Vol. 278, Issue 4, 2228-2235,
January 24, 2003
|
|
|
CNTF triggers weight loss and neurogenesis.
Leptin provides a key feedback signal from peripheral
adipose to two types of neurons in the arcuate nucleus
of the hypothalamus. POMC neurons (brown) are activated
by leptin and inhibit food intake and increase energy
expenditure, whereas AgRP/NPY neurons (blue) have the
opposite response to leptin and the opposite effect on
energy balance. Before CNTF is administered, body fat
has expanded because the key central nervous system targets
of leptin are less responsive. During CNTF treatment,
body weight is lowered because CNTF mimics leptin effects
in the hypothalamus. In addition, CNTF treatment stimulates
the formation of new neurons (orange) involved in weight
regulation, as shown by Kokoeva et al. After CNTF treatment,
these new neurons increase the effects of leptin and thereby
keep adipose mass from returning to its previous levels.
Randy J Seeley. Nature Medicine 11, 1276 - 1278 (2005)
|
|
|
CNTF reduces body weights long term
and induces cell proliferation in the hypothalamus.
(A) Mice were icv infused for 7 days with BrdU (12 µg/day)
in artificial cerebrospinal fluid alone or together
with CNTF (0.75 µg/day) at a flow rate of 12 µl/day.
Body weight (BW) is shown as percentage difference from
initial body weight. All data are mean ± SEM (n = 5
animals per group). (B) BrdU-labeled cells in coronal
sections of the hypothalamus on the level of the arcuate
nucleus. (C) In situ hybridization with a digoxygenin-labeled
probe directed against CNTFR mRNA. Blue precipitate
indicates staining. (D) Fluorescence image of the same
section reveals BrdU+ cells (red). (Insets) High-power
magnification of BrdU+ cells that express CNTFR (arrowheads).
(E) Total number of BrdU+ cells detected in the caudal
hypothalamus of vehicle- and CNTF-infused animals. Brains
were inspected at the indicated times after surgery.
Error bars represent mean ± SEM (n = 3 animals per group).
3V, third ventricle; Arc, arcuate nucleus; Me, median
eminence. Scale bars, 100 µm. Maia V. Kokoeva, Huali
Yin, Jeffrey S. Flier. Science 28 October 2005: Vol.
310. no. 5748, pp. 679 - 683, DOI: 10.1126/science.1115360 |
|
| Newborn hypothalamic cells respond to leptin. Many BrdU+
(red) cells of CNTF-treated mice were also positive for
pSTAT3 (green) after ip leptin injection. (B) 3D confocal
reconstruction of area boxed in (A). (C) Groups of DIO
or ob/ob mice (n = 5) were icv infused for 7 days with
CNTF (0.75 µg/day) or leptin (0.60 µg/day). For all animals,
BrdU (12 µg/day) was coadministered. To induce DIO, mice
were placed on a high-fat diet for 5 months. Body weight
is shown as percentage difference from initial body weight.
All data are mean ± SEM. Scale bars in (A), 50 µm; in
(B), 10 µm. Maia V. Kokoeva, Huali Yin, Jeffrey S. Flier.
Science 28 October 2005: Vol. 310. no. 5748, pp. 679 -
683, DOI: 10.1126/science.1115360 |
|
| In situ hybridization combined with anti-BrdU immunohistochemistry
reveals newborn cells expressing NPY and POMC. Coronal
sections at the level of the arcuate nucleus of a CNTF-treated
mouse 42 days after surgery. (A) Brain section after hybridization
to a digoxigenin-labeled probe for POMC. Inset left: High-power
magnification of a POMC expressing cell (arrow). Inset
right: Fluorescence image of the same cell demonstrating
colocalization with BrdU. (B) Brain section hybridized
to a NPY probe. The NPY-expressing cell marked by an arrow
is also positive for BrdU (Insets). Scale bars: 100 µm.
Maia V. Kokoeva, Huali Yin, Jeffrey S. Flier. Science
28 October 2005: Vol. 310. no. 5748, pp. 679 - 683, DOI:
10.1126/science.1115360 |
|
Schematic representation of hypothalamic
signaling pathways in the regulation of appetite and
energy expenditure. NPY, AgrP, and GABA, the appetite-stimulating
signals, are coproduced in the perikarya located in
the ARC. Likewise, the appetite-inhibiting peptides,
-MSH and CART, are coproduced in the POMC/CART coexpressing
perikarya in the ARC. These distinct populations of
neurons project into the two subdivisions of the PVN,
the mPVN and pPVN, to activate their corresponding receptors
for regulation of appetite and energy expenditure. NPY-producing
neurons also contact the POMC/CART neurons locally in
the ARC to curtail their tonic restraint on appetite.
Leptin inhibits appetite through leptin-Rb located on
the NPY/AgrP/GABA- and POMC/CART-expressing cell bodies
in the ARC and at postsynaptic sites in the PVN where
it regulates release of these signals and enhances energy
expenditure through the sympathetic nervous system.
The results show that CNTF/CNTFAx15, through activation
of the CNTFR located in the ARC and PVN, markedly diminish
the availability of NPY for release in the PVN by suppressing
its synthesis in the ARC and concurrently attenuating
postsynaptic NPYergic signaling by decreasing NPY Y1
receptor and pCREB abundance. Kalra SP. Circumventing
leptin resistance for weight control. Proc Natl Acad
Sci U S A. 2001 Apr 10;98(8):4279-81
|
|
| DR reverses abnormal phenotypes of BDNF+/- mice. Wild-type
(WT) and BDNF+/- mice were maintained for 3 months on
AL or DR feeding regimens. A, Body weights: *, P <
0.05 compared with the WT-AL value; **, P < 0.01 compared
with the corresponding value for AL-fed mice. B, Food
intake: *, P < 0.05 compared with the WT-AL value;
**, P < 0.01 compared with the BDNF+/- AL value. C,
Spontaneous activity: *, P < 0.05 compared with the
WT-AL value; **, P < 0.01 compared with the BDNF+/-
AL value. D, BDNF concentration: *, P < 0.01 compared
with the corresponding value for mice fed AL. All values
are the mean and SEM of determinations made in 8–10
mice per group; statistical comparisons were made using
ANOVA and Scheffé’s post hoc tests. Duan W., et
al. Endocrinology Vol. 144, No. 6 2446-2453 |
|
| Hyperglycemia and impaired glucose tolerance in BDNF+/-
mice are normalized by DR. Wild-type (WT) and BDNF+/-
mice were maintained for 3 months on AL or DR feeding
regimens. A and B, Glucose concentrations were measured
in blood samples taken after an overnight fast (A) or
during feeding conditions (B). Note that the scales for
the glucose concentrations in the two graphs are different.
*, P < 0.01 compared with the value for the same genotype
of mice fed AL; #, P < 0.05 cmpared to the WT-AL value.
C, Mice were administered an oral bolus of glucose (2
g/kg) and the glucose concentration in blood samples taken
at the indicated times was determined. *, P < 0.01
compared with the value for each of the other three groups
at that time point. Values are the mean and SEM of measurements
made in 8–10 mice per group. Statistical comparisons
were made using ANOVA and Scheffé’s post hoc tests.
Duan W., et al. Endocrinology Vol. 144, No. 6 2446-2453 |
|
| Mice with reduced BDNF levels exhibit insulin insensitivity
that is normalized by DR. Wild-type (WT) and BDNF+/- mice
were maintained for 3 months on AL or DR feeding regimens.
A and B, Insulin concentrations were measured in blood
samples taken after an overnight fast (A) or during feeding
conditions (B). *, P < 0.001 compared with the value
for the same genotype of mice fed AL; **, P < 0.001
compared with the WT-AL value. C, Mice were administered
insulin (1 U/kg) and the glucose concentration in blood
samples taken at the indicated times was determined. *,
P < 0.01 compared with the value for each of the other
three groups at that time point. Values are the mean and
SEM of measurements made in 8–10 mice per group.
Statistical comparisons were made using ANOVA and Scheffé’s
post hoc tests. Duan W., et al. Endocrinology Vol. 144,
No. 6 2446-2453 |
|
| gp130 cytokine family pathways on anterior pituitary
cells. Following immune (e.g., LPS) or hormonal (e.g.,
PACAP) stimulation, systemic or anterior pituitary gp130
cytokines, expressed by FS cells among others, induce
the stimulation of ACTH (detailed in the scheme) in the
pituitary. In this pathway, which is distinct from another
mechanism activated by CRH or IL-1, POMC expression and
ACTH secretion are stimulated through STAT3, providing
a new powerful mechanism for regulation of corticotroph
function. Arzt E. J Clin Invest, December 2001, Volume
108, Number 12, 1729-1733 |
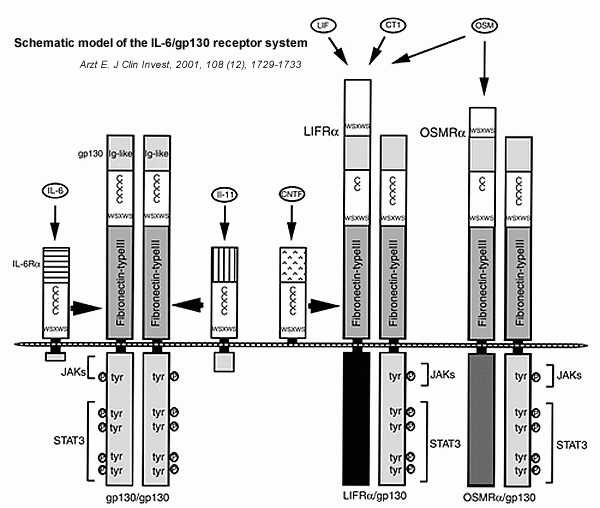 |
| Schematic model of the IL-6/gp130 receptor system. The
specific cytokine-binding subunits and gp130 belong to
a cytokine receptor superfamily characterized by four
positionally conserved cysteine residues and a WSXWS motif.
Functional receptor complexes are induced by the different
gp130 cytokines: IL-6 and IL-11 induce gp130 homodimerization;
CNTF, LIF, CT-1, and OSM induce LIFR/gp130 heterodimers;
and OSM may also induce OSMR/gp130 heterodimers. Following
ligand binding, gp130 is responsible for signal transduction
through the JAK/STAT pathways. |
|
| CNTF does not cause insulin resistance but increases
GLUT 4 expression. A, whole cell extracts were prepared
from fully differentiated 3T3-L1 adipocytes treated with
0.8 nM CNTF for the times shown. Seventy-five µg of each
extract was separated by SDS-PAGE, transferred to nitrocellulose,
and subjected to Western blot analysis. Samples were processed,
and results were visualized as described in Fig. 1 legend.
B, fully differentiated 3T3-L1 adipocytes were treated
with CNTF for 72 h. A fresh bolus of CNTF was added to
the cells every 24 h. Monolayers of adipocytes were used
to examine glucose uptake as indicated under "Experimental
Procedures." This is a representative experiment independently
performed four times. Zvonic S., et al. J. Biol. Chem.,
Vol. 278, Issue 4, 2228-2235, January 24, 2003 |
|
| Time- and dose-dependent effects of CNTF administration
on the phosphorylation and nuclear translocation of STAT
proteins in 3T3-L1 cells. A, cytosolic and nuclear extracts
were prepared from fully differentiated 3T3-L1 adipocytes
following a treatment with 0.8 nM CNTF for the times indicated
at the top of the figure. B, cytosolic and nuclear extracts
were prepared from fully differentiated 3T3-L1 adipocytes
following a 10-min treatment with CNTF at the doses indicated.
C, whole cell extracts were prepared from both preadipocytes
and from fully differentiated 3T3-L1 adipocytes following
a 10-min treatment with CNTF at the doses shown in the
figure. Seventy- five µg of each extract was separated
by SDS-PAGE, transferred to nitrocellulose, and subjected
to Western blot analysis. Samples were processed, and
the results were visualized as described in Fig. 1 legend.
This is a representative experiment independently performed
three times. Zvonic S., et al. J. Biol. Chem., Vol. 278,
Issue 4, 2228-2235, January 24, 2003 |
|
The effects of acute CNTF administration on the expression
of adipocyte proteins. Whole cell extracts were prepared
from fully differentiated 3T3-L1 adipocytes treated with
0.8 nM CNTF for the times shown. Seventy-five µg of each
extract was separated by SDS-PAGE, transferred to nitrocellulose,
and subjected to Western blot analysis. Samples were processed
and results were visualized as described in Fig. 1 legend.
This is a representative experiment independently performed
three times.
Zvonic S., et al. J. Biol. Chem., Vol. 278, Issue 4, 2228-2235,
January 24, 2003 |
|
| The effects of chronic CNTF administration on the expression
of adipocyte proteins. Whole cell extracts were prepared
from fully differentiated 3T3-L1 adipocytes treated with
0.8 nM CNTF for the times indicated at the top of the
figure. Seventy-five µg of each extract was separated
by SDS-PAGE, transferred to nitrocellulose, and subjected
to Western blot analysis. Samples were processed, and
results were visualized as described in Fig. 1 legend.
This is a representative experiment independently performed
three times. Zvonic S., et al. J. Biol. Chem., Vol. 278,
Issue 4, 2228-2235, January 24, 2003 |
|
In vivo effect of acute CNTF administration
in rodents. Six-week-old C57Bl/6J mice were given an
intraperitoneal injection of CNTF (33.3 µg/kg) or vehicle
(saline) control. Fifteen minutes after the injection,
the mice were sacrificed, and epididymal fat pads, brains,
and skeletal muscle were immediately removed and frozen
in liquid nitrogen. Tissue extracts were analyzed from
epididymal fat pads (A) and brain and skeletal muscle
(B). In each panel, 75 µg of each extract was separated
by SDS-PAGE, transferred to nitrocellulose, and subjected
to Western blot analysis. Samples were processed, and
results were visualized as described in Fig. 1 legend.
This is a representative experiment independently performed
two times. Zvonic S., et al. J. Biol. Chem., Vol. 278,
Issue 4, 2228-2235, January 24, 2003 |
|
In vivo expression of CNTFR in epididymal fat pads. Epididymal
fat pads were extracted from 6-week-old lean C57Bl/6J
mice and fractionated into adipocyte and stromovascular
fractions. Seventy-five µg of each extract was separated
by SDS-PAGE, transferred to nitrocellulose, and subjected
to Western blot analysis. Samples were processed, and
results were visualized as described in Fig. 1 legend.
Zvonic S., et al. J. Biol. Chem., Vol. 278, Issue 4, 2228-2235,
January 24, 2003
|
|


