LEAP-2
A New Liver-expressed
Antimicrobial Peptide 2
Isolation and biochemical characterization of
LEAP-2, a novel blood
peptide expressed in the liver
The human genome
contains numerous genes whose protein products are unknown in terms of
structure, interaction partner, expression, and function. To unravel the
function of these orphan genes, it is of particular value to isolate native
forms of protein and peptide products derived from these genes. From human blood
ultrafiltrate, we characterized a novel gene-encoded, cysteine-rich, and
cationic peptide that we termed liver-expressed antimicrobial peptide 2
(LEAP-2). We identified
several circulating forms of LEAP-2 differing in their
amino-terminal length, all containing a core structure with two disulfide bonds
formed by cysteine residues in relative 1–3 and 2–4 positions. Molecular cloning
of the cDNA showed that LEAP-2 is synthesized as
a 77-residue precursor, which is predominantly expressed in the liver and highly
conserved among mammals. This makes it a unique peptide that does not exhibit
similarity with any known human peptide regarding its primary structure,
disulfide motif, and expression. Analysis of the LEAP-2 gene resulted in
the identification of an alternative promoter and at least four different
splicing variants, with the two dominating transcripts being tissue-specifically
expressed. The largest native LEAP-2 form of 40 amino
acid residues is generated from the precursor at a putative cleavage site for a
furin-like endoprotease. In contrast to smaller LEAP-2 variants, this
peptide exhibited dose-dependent antimicrobial activity against selected
microbial model organisms. LEAP-2 shares some
characteristic properties with classic peptide hormones and it is expected that
the isolation of this novel peptide will help to unravel its physiological
role.
Krause A., et al.
Protein Science (2003), 12:143-152
LEAP-2
immunohistochemistry in liver and kidney tissue




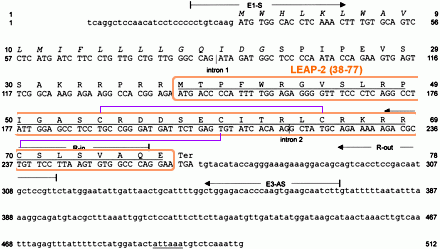
Human LEAP-2
precursor cDNA and deduced amino acid sequence. Coding regions are printed in
capital letters. The typical secretory signal sequence 1–22 is printed in
italics, whereas the positions of introns 1 (198 bp) and 2 (320 bp) of the
LEAP-2 gene are marked by vertical lines. Two TATAAA sequences located 31 and 84
bases upstream of the translational start were identified. The putatively mature
and antimicrobially active LEAP-2-(38–77) isolated from hemofiltrate and the
putative polyadenylation signal are underlined. The position of the primers used
for nested 5'-RACE-PCR and for preparative PCR from six different species is
indicated.

Alignment ofLEAP-2 from mammalian
species. Standard PCR carried out with primers originally designed for the human
LEAP-2 gene revealed homologous LEAP-2 forms in rhesus monkey, cow, pig, mouse,
and guinea pig. The depicted putative peptide sequences are deduced from the
cDNA sequences obtained. Underlined amino acids represent the putative signal
peptide sequences as predicted by the SignalP V2.0 program (Nielsen et al.
1997), and the mature LEAP-2 (38–77) form is hyphenated. The nucleotide sequence
data reported in this paper have been submitted to the GenBank/EBI Data Bank
with accession numbers AJ306405 (Homo sapiens mRNA), AJ409013 (Sus scrofa mRNA),
AJ409014 (Bos taurus mRNA), AJ409054 (Cavia porcellus mRNA), AJ409055 (Mus
musculus mRNA), AJ409056 (Macaca mulatta genomic DNA), AJ409063 (Mus
musculus genomic DNA), AJ409064 (Homo sapiens genomic DNA), and AJ409065 (Homo
sapiens alternative promoter sequence).
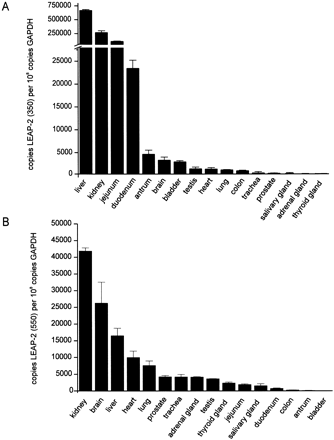
Tissue distribution
of the two main LEAP-2 transcripts.
Expression analysis of the LEAP-2 gene was performed
by real-time quantitative RT-PCR specific for LEAP-2350 (A) and LEAP-2550 (B)
transcripts. Standard curves were constructed using a reference plasmid
representing a known number of copies of the target gene as well as the
housekeeping gene. Expression levels are therefore depicted as number of copies
of LEAP-2 transcript per 108 copies of the housekeeping gene GAPDH. Experiments
were performed in duplicate at least twice.
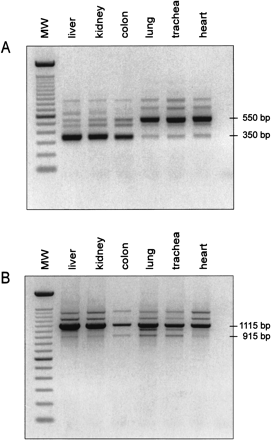
Alternative splicing
in the LEAP-2 gene. (A) Standard
PCR conducted with the primer combination E1-S and E3-AS (Fig. 2) and 20 ng of
DNase-treated cDNA. LEAP-2350 is mainly
expressed in liver, kidney, and colon, whereas LEAP-2550 is the main transcript
in lung, trachea, and heart. PCR products at 650 bp, 720 bp, and 870 bp
represent splicing variants of LEAP-2, and the band at 480 bp could be
identified as a PCR artefact. (MW = marker, 100 bp DNA ladder, Life
Technologies). (B) Standard PCR performed with the primers PROM-S (5'-GGTGCA
GATTAGGGTGACAGTCCATC-3'), which is located 565 bp upstream of the
transcriptional start depicted in Figure 2, and E3-AS. Lung, heart, and trachea
exhibit the same pattern of bands, including the 565 bp shift in size caused by
the upstream primer PROM-S. The main transcript identified in liver, kidney, and
colon, however, changes from the completely spliced LEAP-2350 to the intron
1-retaining LEAP-2 variant. All bands obtained were characterized by DNA
sequencing.
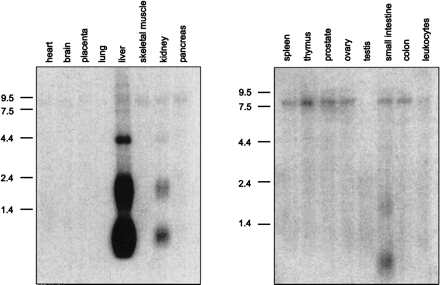
Northern Blot
analysis. Human MTN Blots I+II (Clontech, A+B) were hybridized under
high-stringency conditions with a 32P-labeled LEAP-2-specific cDNA fragment. A
transcript size of 0.7 kb identified in liver, kidney, and small intestine
represents the complete LEAP-2 cDNA including a
poly(A)-tail of 200 bp. The signal at 2.0 kb corresponds to the LEAP-2 form
coded by the distal promoter, which could also be identified in 5'-RACE-PCR,
whereas the bands at 4.2 kb and 8.0 kb might either represent additional
alternative promoter variants or homologous proteins related to
LEAP-2.
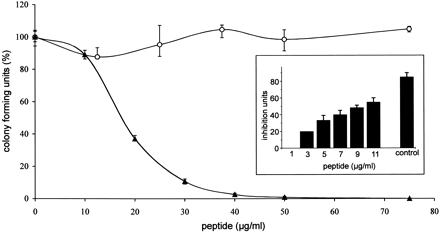
Antimicrobial
activity of LEAP-2-(38–77) and
LEAP-2-(44–77).
Colony-forming unit assay of synthetic LEAP-2-(38–77) (solid
triangles) and LEAP-2-(44–77) (open
circles), the two main peptide forms isolated from hemofiltrate, against S.
cerevisiae ATCC9763. Incubation without peptide represents 100% CFU. Synthetic
and native LEAP-2-(44–77) led to
similar results. The bars indicate the minimum and maximum value of the
triplicates used in this representative assay. The inset depicts the
dose-dependent effect of LEAP-2-(38–77) against S. cerevisiae in a radial
diffusion assay. LEAP-2-(44–77) showed no effect in this sensitive antimicrobial
assay. The antimicrobially active casocidin-I (11 µg/well) served as a positive
control ( Zucht et al. 1998). One inhibition unit (1 IU) corresponds to
0.1 mm diameter of growth inhibition zone, and the error bars represent the S.D.
calculated from three experiments performed.


