Neuropeptide FF Receptor 1 & 2 (NPFF-1R & -2R)---GPCR for Neuropeptide FF (NPFF)
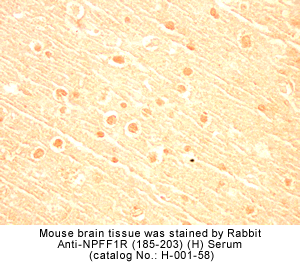
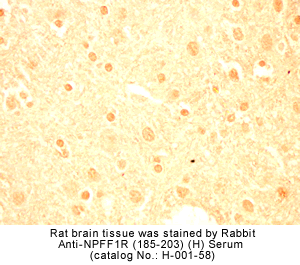
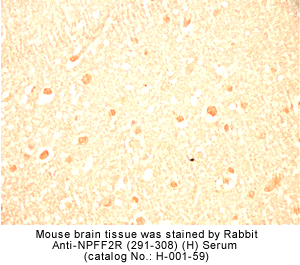
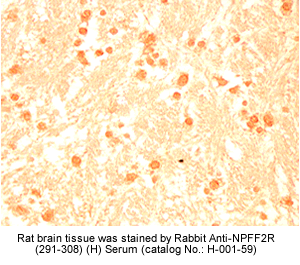
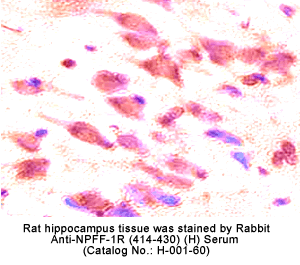
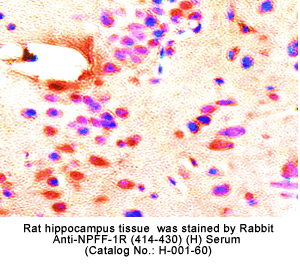
Identification and characterization of two G protein-coupled receptors for neuropeptide FF
The central nervous system octapeptide, neuropeptide FF (NPFF), is believed to play a role in pain modulation and opiate tolerance.
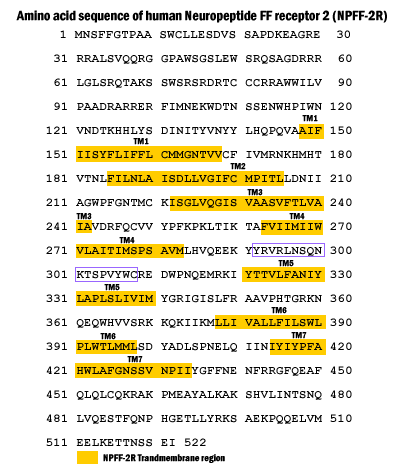
Two G protein-coupled receptors, NPFF1 and NPFF2, were isolated from human and rat central nervous system tissues.
NPFF specifically bound to NPFF1 (K(d) = 1.13 nm) and NPFF2 (K(d) = 0.37 nm), and both receptors were activated by NPFF in a variety of heterologous expression systems.
The localization of mRNA and binding sites of these receptors in the dorsal horn of the spinal cord, the lateral hypothalamus, the spinal trigeminal nuclei, and the thalamic nuclei supports a role for NPFF in pain modulation.
Among the receptors with the highest amino acid sequence homology to NPFF1 and NPFF2 are members of the orexin, NPY, and cholecystokinin families, which have been implicated in feeding.
These similarities together with the finding that BIBP3226, an anorexigenic Y1 receptor ligand, also binds to NPFF1 suggest a potential role for NPFF1 in feeding.
The identification of NPFF1 and NPFF2 will help delineate their roles in these and other physiological functions.
Bonini JA, et al. J Biol Chem 2000 Dec 15;275(50):39324-31
Receptor for the pain modulatory
neuropeptides FF and AF is an orphan G protein-coupled receptor
Opiate tolerance and dependence are major clinical and social problems. The anti-opiate neuropeptides FF and AF (NPFF and NPAF) have been implicated in pain modulation as well as in opioid tolerance and may play a critical role in this process, although their mechanism of action has remained unknown.
Here we describe a cDNA encoding a novel neuropeptide Y-like human orphan G protein-coupled receptor (GPCR), referred to as HLWAR77 for which NPAF and NPFF have high affinity.
Cells transiently or stably expressing HLWAR77 bind and respond in a concentration-dependent manner to NPAF and NPFF and are also weakly activated by FMRF-amide (Phe-Met-Arg-Phe-amide) and a variety of related peptides. The high affinity and potency of human NPFF and human NPAF for HLWAR77 strongly suggest that these are the cognate ligands for this receptor.
Expression of HLWAR77 was demonstrated in brain regions associated with opiate activity, consistent with the pain-modulating activity of these peptides, whereas the expression in adipose tissue suggests other physiological and pathophysiological activities for FMRF-amide neuropeptides.
The discovery that the anti-opiate neuropeptides are the endogenous ligands for HLWAR77 will aid in defining the physiological role(s) of these ligands and facilitate the identification of receptor agonists and antagonists.
Elshourbagy
NA, et al. J Biol Chem 2000 Aug 25;275(34):25965-71
Pharmacological
characterization of human NPFF(1) and NPFF(2) receptors expressed in CHO
cells by using NPY Y(1) receptor antagonists
Neuropeptide FF (NPFF) belongs to an opioid-modulatory
system including two precursors (pro-NPFF(A) and pro-NPFF(B)) and two G-protein
coupled receptors (NPFF(1) and NPFF(2)).
The pharmacological and functional
profiles of human NPFF(1) and NPFF(2) receptors expressed in Chinese hamster
ovary (CHO) cells were compared by determining the affinity of several peptides
derived from both NPFF precursors and by measuring their abilities to inhibit
forskolin-induced cAMP accumulation.
Each NPFF receptor recognizes peptides
from both precursors with nanomolar affinities, however, with a slight preference
of pro-NPFF(A) peptides for NPFF(2) receptors and of pro-NPFF(B) peptides for
NPFF(1) receptors. BIBP3226 ((R)-N(2)-(diphenylacetyl)-N-[(4-hydroxyphenyl)-methyl]-argininamide)
and BIBO3304 ((R)-N(2)-(diphenylacetyl)-N-[4-(aminocarbonylaminomethyl)-benzyl]-argininamide
trifluoroacetate), two selective neuropeptide Y (NPY) Y(1) receptor antagonists,
display relative high affinities for NPFF receptors and exhibit antagonist
properties towards hNPFF(1) receptors.
The structural determinants responsible
for binding of these molecules to NPFF receptors were investigated and led
to the synthesis of hNPFF(1) receptor antagonists with affinities from 40 to
80 nM. Our results demonstrate differences in pharmacological characteristics
between NPFF(1) and NPFF(2) receptors and the feasibility of subtype-selective
antagonists.
Mollereau C, et al. Eur J Pharmacol 2002 Sep 20;451(3):245
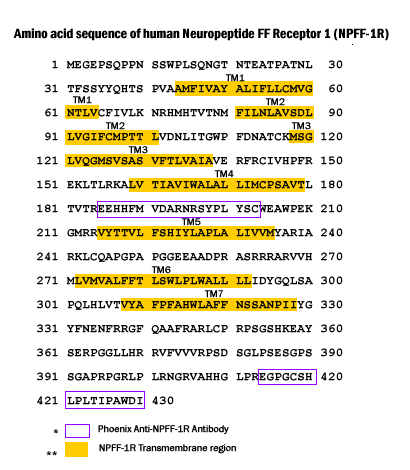
Peptides
stimulated Ca2+ mobilization response in HLWAR77 expressed HEK-293
cells
RT-PCR
analysis of mRNA levels in human tissues
| Tissue |
mRNA of HLWAR77 |
CNS |
++ |
Adipose |
+++ |
Placenta |
+++++ |
Amygdala |
++++ |
Cingulate gyrus |
++++++ |
Nucleus accumbers |
+++ |
Superior frontal gyrus |
+++ |


