Vasohibin
A
novel angiogenesis inhibitor
Gene regulation of a novel
angiogenesis inhibitor, vasohibin, in endothelial
cells
We recently reported that vasohibin is a negative feedback regulator of
angiogenesis, and it is specifically expressed in endothelial cells. Here,
we characterize the regulation of vasohibin expression. Two possible
splicing variants were found, and the longer isoform was preferentially
expressed. VEGF induced the expression of vasohibin, and this induction
was abrogated by anti-VEGFR2 mAb but not by anti-VEGFR1 mAb.
Pharmacological analysis revealed that the downstream targets of VEGFR2
were PKCs, especially PKCdelta. Actinomycin D did not alter the kinetics
of vasohibin mRNA induction upon VEGF treatment, whereas cycloheximide
completely abolished its induction. We tested the effect of various
inflammatory cytokines on vasohibin expression. TNFalpha, IL1 and IFNgamma
decreased VEGF-stimulated vasohibin expression. Actinomycin D did not
alter the kinetics of vasohibin mRNA induction upon TNFalpha treatment.
These results indicate that the expression of vasohibin in endothelial
cells is regulated either positively or negatively by certain factors at
the transcriptional level.
Shimizu K, et al. PBiochem Biophys Res
Commun. 2005 Feb 18;327(3):700-6
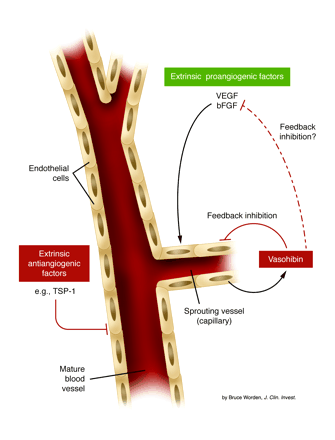
Control of angiogenesis by the
action of extrinsic angiogenesis inhibitors (antiangiogenic factors), an
intrinsic endothelial cell inhibitor (vasohibin), and extrinsic
stimulators of angiogenesis such as VEGF and bFGF. Many of the extrinsic
type inhibitors, some of which are listed in Table 1,
may act to keep established mature blood vessels in a quiescent state
(left side of the diagram); they may also contribute to pathologic,
sprouting angiogenesis as a result of their downregulation and/or
suppression, thus allowing stimulators such as VEGF or bFGF to act more
efficiently. The intrinsic inhibitor, vasohibin, is induced in endothelial
cells at later stages of sprouting vessel formation and acts in some
fashion as a feedback mechanism to limit excessive angiogenesis (right
side of the diagram), e.g., by directly interacting with endothelial cells
in sprouting vessels or perhaps by direct interaction with and
neutralization of the stimulator(s) which induced vasohibin in endothelial
cells. Robert S. Kerbel. J. Clin.
Invest. 114:884-886
(2004).
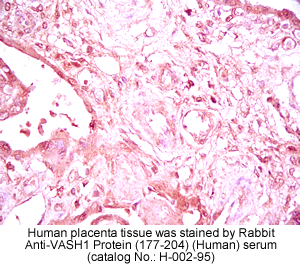
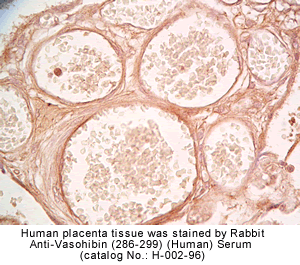
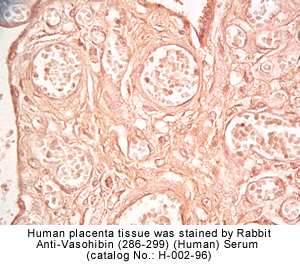
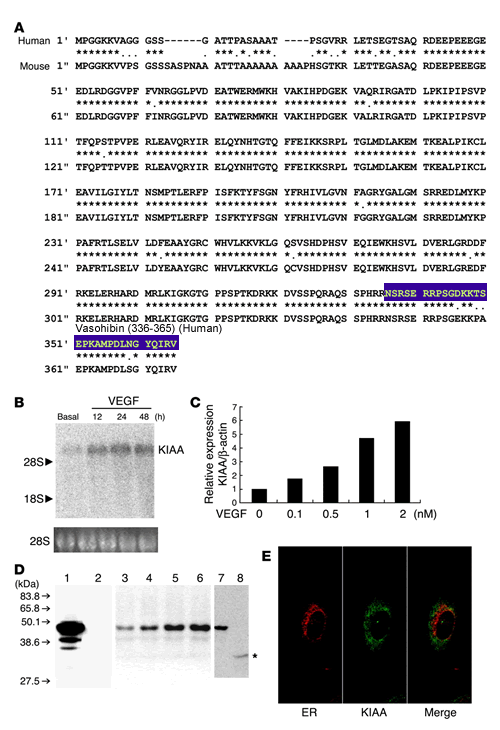
KIAA1036 is an
endothelium-derived VEGF-inducible secretory protein. (A) The deduced amino acid
sequences of the human and mouse KIAA1036 (KIAA) proteins are shown.
Asterisks indicate identical amino acids between human and mouse.
(B) A single
KIAA1036 mRNA was induced by VEGF. HUVECs were stimulated with VEGF (1 nM)
for the indicated periods and then Northern blotting was performed.
(C) VEGF
increased KIAA1036 mRNA in a concentration-dependent manner. HUVECs were
stimulated with the indicated concentration of VEGF for 24 hours and then
real-time RT-PCR was performed. (D) KIAA1036 protein was
synthesized and secreted. GM7373 cells transfected with KIAA1036 gene were
lysed. Equal amounts of protein were applied to lane 1 and lane 2, and
transferred to the filter. The filter was then separated into 2 parts.
Western blotting was performed with anti_KIAA1036 mAb (lane 1). Prior to
Western blotting, anti_KIAA1036 mAb was absorbed with antigen peptide
(lane 2). HUVECs were stimulated with VEGF (1 nM) for the following
periods and were lysed for Western blotting: lane 3, 0 hours; lane 4, 12
hours; lane 5, 24 hours; lane 6, 48 hours. HUVECs were cultured for 3 days
in the growth medium and then cells were lysed for Western blotting (lane
7). After this incubation, the medium was collected and concentrated. Five
hundred microliters of concentrated medium was subjected to
immunoprecipitation followed by Western blotting (lane 8). Asterisk
indicates protein in the medium. (E) KIAA1036 protein does not
colocalize with ER. HUVECs in the growth medium were used for the
immunostaining of calnexin (red) and KIAA1036 protein (green). Watanabe
K., et al. J. Clin.
Invest. 114:898-907
(2004)
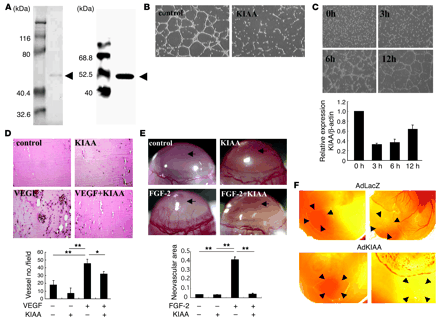
KIAA1036 inhibits angiogenesis. (A) Preparation of KIAA1036
protein. SDS-PAGE/Coomassie brilliant blue staining is shown on the left
and Western blotting on the right. Arrows indicate KIAA1036.
(B) Effect of
KIAA1036 on network formation by ECs. HUVECs were plated on Matrigel in
the absence or presence of KIAA1036 (10 nM). (C) Downregulation of KIAA1036
during network formation on Matrigel. HUVECs were plated on Matrigel, and
after the indicated period of incubation, total RNA was obtained and
real-time RT-PCR was performed. Values are expressed as mean ± SD of 3
samples. (D)
Matrigel implantation analysis was performed as described in Methods. The
vessel number per low-power field in 3 different fields was counted for
each sample. Values are expressed as mean ± SD of 5 animals.
(E) Mouse
corneal micropocket assay was performed as described in Methods. Arrows
indicate the site where pellets were implanted. Neovascular area (mm2) was
determined using NIH Image. Values are expressed as mean ± SD of 5 eyes.
(F) CAM assay
using adenovirus vectors was performed as described in Methods. The nylon
mesh containing adenovirus/Matrigel mixture was placed on the peripheral
zone of the CAM, where vascular structure would not appear (left panels).
Four days after adenovirus infection, vascular formation was evaluated by
macroscopic observation (right). Arrowheads indicate the site where the
nylon mesh was placed. *P < 0.05, **P < 0.01. Watanabe K., et al. J. Clin. Invest. 114:898-907
(2004)
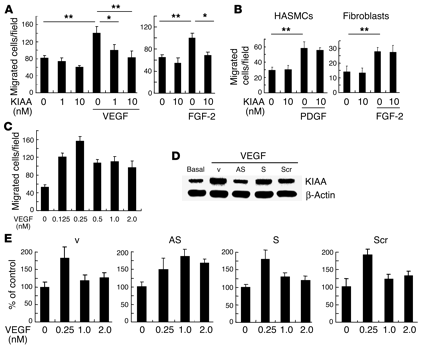
KIAA1036 may act as a negative feedback regulator. (A) Effect of KIAA1036 on the
migration of HUVECs. Migration of HUVECs was analyzed as described in
Methods. The indicated concentrations of growth factors and/or KIAA1036
protein were placed in the lower chamber of the Transwell insert. Values
are expressed as mean ± SD of 4 samples. (B) Effect of KIAA1036 on the
migration of HASMCs or fibroblasts. Migration of HASMCs or fibroblasts was
analyzed as described in Methods. The indicated combinations of PDGF,
FGF-2, and KIAA1036 protein were placed in the lower chamber. Values are
expressed as mean ± SD of 4 samples. (C) Bell-shaped pattern of the
VEGF-stimulated migration of HUVECs. The indicated concentrations of VEGF
were placed in the lower chamber and HUVECs were plated in the upper
chamber. Values are expressed as mean ± SD of 4 samples. (D) Selective downregulation
of KIAA1036 synthesis. HUVECs were incubated for 4 hours with 500 nM
synthetic phosphorothioate ODNs or vehicle alone. Thereafter, HUVECs were
stimulated with VEGF (1 nM) for 24 hours, and Western blotting for
KIAA1036 was performed. AS, AS-ODN; S, S-ODN; Scr, Scr-ODN; v, vehicle.
(E) Modulation
of the bell-shaped pattern of the VEGF effect by KIAA1036 AS-ODNs. HUVECs
were incubated for 4 hours with 500 nM phosphorothioate ODNs. Thereafter,
HUVECs were subjected to the migration assay described above. Values are
expressed as mean ± SD of 4 samples. *P < 0.05, **P < 0.01. Watanabe K., et al. J. Clin. Invest. 114:898-907
(2004)
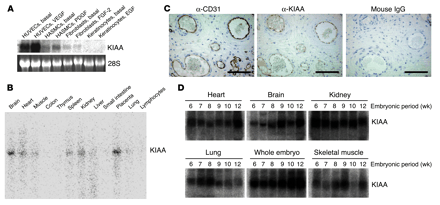
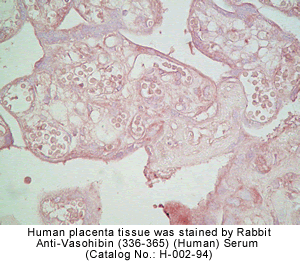
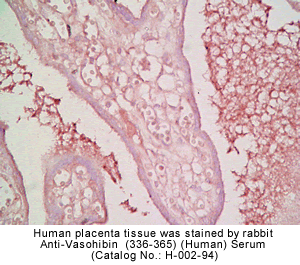
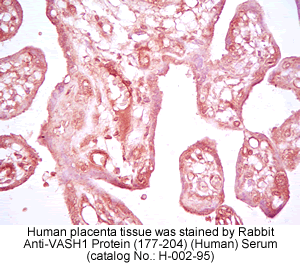
KIAA1036 is preferentially
expressed in ECs. (A) Expression of KIAA1036 in
cultured cells. Cells were preincubated in 0.1% FCS/alpha-MEM for 12 hours
and then stimulated with growth factors as follows: HUVECs with VEGF (1
nM), HASMCs with PDGF (1 nM), human fibroblasts with FGF-2 (2 nM), and
keratinocytes with EGF (1 nM). Thereafter, total RNA was obtained and
Northern blotting for vasohibin was performed. (B) Expression of KIAA1036 in
vivo was examined by multiple-tissue Northern blot. (C) Localization of KIAA1036
protein in the placenta. Sections of human placenta were subjected to
immunostaining. Anti_human CD31 mAb, anti_KIAA1036 mAb, or mouse IgG was
used as the primary Ab. Scale bars: 100 µm. (D) Expression of KIAA1036 in
human embryo. Northern blotting for vasohibin was performed using a human
developmental total RNA Northern blot. Watanabe K., et al. J. Clin. Invest. 114:898-907
(2004)
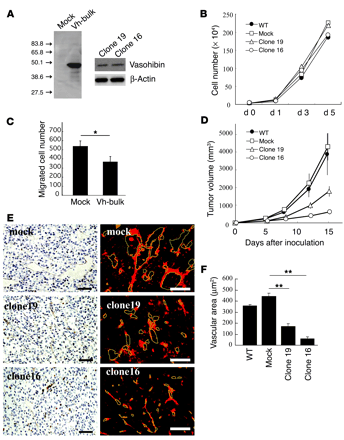
Vasohibin suppresses tumor
growth and tumor angiogenesis. (A) The synthesis of vasohibin
protein in LLC cells. Cell extracts were prepared from mock or vasohibin
transfectants (Vh-bulk) for Western blotting. Clone 16 and clone 19 were
vasohibin-producing clones. (B) Effect of vasohibin on the
proliferation of LLC cells in vitro. Proliferation of mock transfectants,
vasohibin transfectants, clone 16, and clone 19 was determined.
(C) Effect of
secreted vasohibin from LLC cells on the migration of HUVECs. Mock or
vasohibin transfectants were plated on the lower compartment of a modified
Boyden chamber and the migration of HUVECs toward the lower chamber of the
Transwell insert was analyzed. Values are expressed as mean ± SD of 4
samples. (D)
Effect of vasohibin gene transfection on the growth of LLC cells in vivo.
BDF1 mice were inoculated intradermally with LLC cells. Tumor volume was
determined consecutively. (E) Effect of vasohibin gene
transfection on tumor angiogenesis. Paraffin sections were prepared from
tumors for the immunostaining of CD31; sections obtained on day 8 after
inoculation are shown. Visualization with a DAKO LSAB+/HRP kit is shown at left, and that
with streptavidin-Cy3 conjugate on the right. Yellow lines trace vascular
lumens. Scale bars: 50 µm. (F) Quantitative analysis of
tumor vascular area. Total vascular area per field was determined using
NIH Image and compared. Values are expressed as mean ± SD of 6 random
fields. *P < 0.05;
**P < 0.01. Watanabe
K., et al. J. Clin.
Invest. 114:898-907
(2004)
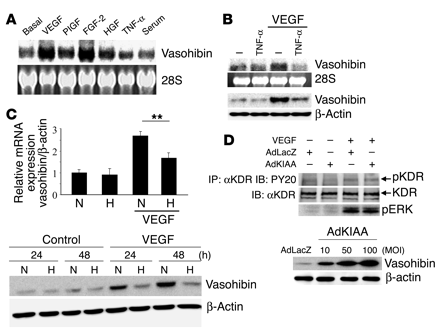
Modulation of vasohibin
expression and the effect of vasohibin on VEGF-stimulated signaling in
HUVECs. (A)
Induction of vasohibin. HUVECs were stimulated with VEGF (1 nM), PlGF (1
nM), FGF-2 (2 nM), HGF (1 nM), TNF-alpha (1 nM), or 10% serum for 24
hours. Thereafter, total RNA was obtained and Northern blotting for
vasohibin was performed. (B) Effect of TNF-alpha on the
induction of vasohibin by VEGF. HUVECs were stimulated with VEGF (1 nM)
and/or TNF-alpha (1 nM). Thereafter, Northern blotting and Western
blotting for vasohibin were performed. (C) Effect of hypoxia on the
induction of vasohibin by VEGF. HUVECs were stimulated with VEGF (1 nM)
under normoxic (N) or hypoxic (H) conditions. Upper panel: Total RNA was
obtained and real-time RT-PCR of vasohibin was performed. Values are
expressed as mean ± SD of 4 samples. **P < 0.01. Lower panel: Cell
extract was obtained and Western blotting for vasohibin was performed.
(D) Effect of
vasohibin on VEGF-mediated KDR tyrosine phosphorylation or ERK1/2
activation of HUVECs. HUVECs were infected with AdLacZ or AdKIAA at an MOI
of 100, and then stimulated with VEGF (10 ng/ml). VEGF-mediated KDR
tyrosine phosphorylation or ERK1/2 activation was analyzed. Results shown
in lower panel indicate that AdKIAA increased the synthesis of vasohibin
in an MOI-dependent manner. IP, immunoprecipitation; IB, immunoblotting;
pKDR, phosphorylated KDR. Watanabe K., et al. J. Clin. Invest. 114:898-907
(2004)


